Groundbreaking Alzheimer’s Therapies: From Pipeline to Patient Care
Apr 18, 2025
Table of Contents
The Alzheimer’s and Parkinson’s Diseases (AD/PD) 2025 International Conference, conducted in April in Vienna, Austria, and online, stands as a pivotal event in neurodegenerative disease research. This hybrid gathering brings together global experts to delve into the latest advancements in Alzheimer’s and Parkinson’s diseases, emphasizing breakthroughs in treatment, translational research, early diagnosis, and clinical trials. By fostering interdisciplinary collaboration and highlighting the convergence of disease mechanisms, AD/PD 2025 aims to accelerate the development of innovative therapies and improve patient outcomes. The conference’s commitment to integrating basic science with clinical application underscores its role in shaping the future of neurodegenerative disease management.
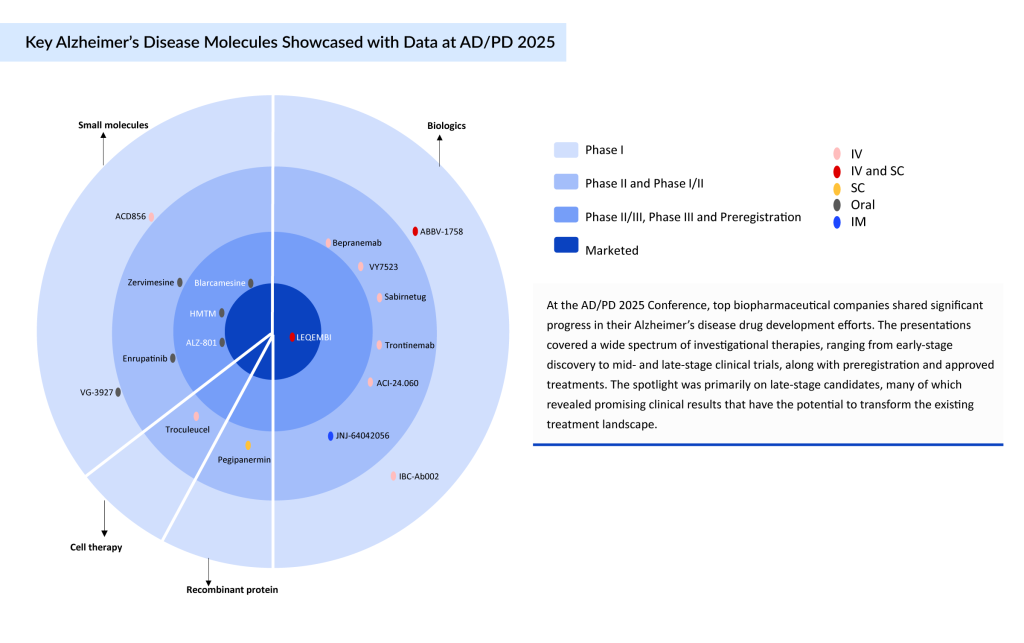
At the AD/PD 2025 Conference, leading biopharmaceutical companies presented key updates on their Alzheimer’s disease drug development programs. A broad range of investigational therapies was discussed, spanning early discovery to mid- and late-stage clinical trials, as well as preregistration and marketed products. Notably, late-stage candidates drew the most attention, as several showed compelling clinical data that could reshape the current treatment landscape.
Downloads
Article in PDF
Recent Articles
- Exploring the Current Alzheimer’s Disease Drug Development Pipeline
- Does coconut oil help prevent dementia?
- Bristol Myers Squibb’ Karuna Therapeutics Buyout; Orchard Therapeutics’ Lenmeldy FDA Approval; Ma...
- Zealand Pharma’s Phase III Results of Glepaglutide; FDA Approves Amylyx’s ALS Drug Relyvrio; Novo...
- Alzeimer’s drug; Guardant Health receives approval; First Patient Dosed in Phase 1 Clinical Trial...
These advanced-stage therapies, targeting mechanisms such as amyloid and tau pathology, neuroinflammation, and synaptic protection, demonstrated promising efficacy and safety outcomes. Their development reflects a growing shift from symptomatic relief toward disease-modifying strategies, offering renewed hope for patients and caregivers.
Highlights from the Alzheimer’s and Parkinson’s Diseases 2025 International Conference
The conference highlighted a critical moment in Alzheimer’s research as the pipeline continues to mature with multiple therapies nearing regulatory review. These advancements signal a potential turning point in the fight against Alzheimer’s, with real-world impacts expected in the near future.
TauRx’s A Second Chance at Changing Alzheimer’s Treatment
At the AD/PD 2025 Conference, TauRx Pharmaceuticals reignited interest in its investigational drug Hydromethylthionine Mesylate (HMTM), presenting compelling late-stage data that may mark a turning point in Alzheimer’s treatment. Unlike most currently approved therapies that target amyloid plaques, HMTM is an oral tau aggregation inhibitor, aiming to disrupt the abnormal buildup of tau protein—a critical driver of neurodegeneration in Alzheimer’s disease.
In updated findings from the Phase III LUCIDITY trial, HMTM demonstrated sustained clinical benefit in patients with early to moderate Alzheimer’s, including slowed cognitive decline and brain atrophy over 18 months, with some effects extending up to 2 years. The drug also exhibited a favorable safety profile across more than 3,000 participants, and its once-daily oral formulation positions it as a practical, low-burden therapy for long-term use.
TauRx has already submitted a Marketing Authorisation Application (MAA) to the UK’s MHRA and is working with the National Institute for Health and Care Excellence (NICE) to explore the drug’s integration into NHS treatment pathways. Plans are underway to seek regulatory approval in the US and Canada in 2026.
Yet, HMTM’s journey has not been without setbacks. The compound, previously known as LMTM or LMTX, failed a pivotal Phase III trial in 2016, casting doubt on its viability. Though the new LUCIDITY trial results appear promising, they lack a traditional placebo-controlled comparison, which may be a stumbling block when facing regulatory agencies like the US FDA.
To address this, TauRx supplemented its findings with long-term follow-up data and comparative analyses using external placebo benchmarks from the Critical Path for Alzheimer’s Disease (CPAD) database. These analyses suggest that HMTM may significantly slow disease progression when used as monotherapy in early Alzheimer’s—an important distinction as the company continues building a case for approval. Whether this alternative approach to tau-targeting therapy will persuade global regulators remains to be seen. But with its differentiated mechanism, strong safety record, and convenient oral dosing, HMTM offers renewed hope for a scalable, disease-modifying Alzheimer’s treatment—and a second chance for a molecule that once seemed destined for the shelf.
Turning Setbacks into Strategy: Valiltramiprosate
Valiltramiprosate (ALZ-801), an oral small molecule designed to block the formation of neurotoxic soluble Amyloid Beta (Aβ) oligomers, represents a precision medicine approach in Alzheimer’s disease therapy. Originally developed by Neurochem and later acquired by Alzheon from Bellus Health in 2013, the drug has been revitalized with a focus on genetically defined populations.
The pivotal Phase III APOLLOE4 trial, which targeted early-stage Alzheimer’s patients homozygous for the APOE4 gene (APOE4/4), did not meet its primary cognitive endpoint in the overall study population. However, a prespecified subgroup analysis revealed a notable benefit in patients with Mild Cognitive Impairment (MCI), where ALZ-801 demonstrated a statistically significant 52% reduction in cognitive decline, accompanied by improvements in functional measures. These findings support the idea that early intervention in high-risk APOE4/4 carriers may yield meaningful therapeutic outcomes. In recognition of its potential to address a critical unmet need, the FDA granted ALZ-801 Fast Track Designation (FTD), highlighting its promise as a targeted, disease-modifying treatment.
Neuroimaging results further reinforced the clinical data, showing reductions in hippocampal atrophy and cortical thinning—changes that correlated with preserved cognitive performance. These outcomes have guided a strategic shift in the drug’s development, prioritizing early-stage MCI patients who carry two copies of the APOE4 gene. Currently, the Phase III program is focused on this genetically high-risk group, but future expansion is planned to include individuals with one copy of the APOE4 gene (heterozygotes) and even noncarriers. If ongoing research continues to validate these findings, ALZ-801 could emerge as the first oral, disease-modifying therapy specifically tailored to genetic risk, potentially ushering in a new era of precision, preventative treatment in Alzheimer’s disease.
No ARIA, No Deaths, Just Results: Blarcamesine’s Strong Profile
At the AD/PD 2025 conference, Anavex Life Sciences unveiled compelling long-term results for blarcamesine (ANAVEX 2-73), an oral small molecule targeting Sigma-1 Receptor (SIGMAR1) to restore autophagy and counter neurodegeneration in early Alzheimer’s disease. In the ongoing Phase IIb/III ATTENTION-AD open-label extension trial, up to 4 years of continuous treatment demonstrated sustained cognitive and functional benefits. The prespecified delayed-start analysis showed statistically significant and clinically meaningful advantages for patients who began treatment earlier: ADAS-Cog13 scores improved by -3.83 (p = 0.0165) and ADCS-ADL by +4.30 (p = 0.0206) at Week 192. These findings support a potential disease-modifying effect, especially when treatment is initiated early and maintained without interruption.
Blarcamesine’s favorable safety profile stood out, with no ARIA-related imaging abnormalities or drug-related deaths. Adverse events were mostly mild and transient, such as dizziness during titration. Additionally, biomarker data revealed that SIGMAR1 wild-type carriers (~70% of the population) experienced nearly 50% greater cognitive benefit compared to the overall cohort, emphasizing its precision medicine potential.
With its convenient once-daily oral dosing, absence of severe safety concerns, and long-term efficacy, blarcamesine may offer a scalable, patient-friendly therapeutic option. These data solidify its promise as a novel, disease-modifying approach for managing early Alzheimer’s disease.
LEQEMBI’s Clinical Durability
At AD/PD 2025, lecanemab (LEQEMBI)—the first fully approved anti-amyloid-beta monoclonal Antibody (mAb) in the US—stood out as a foundational therapy reshaping the Alzheimer’s treatment landscape. Developed by Eisai and BioArctic, lecanemab was reinforced by a growing body of clinical and real-world evidence supporting its sustained efficacy and favorable safety profile in early Alzheimer’s disease, particularly among ApoE ε4 heterozygous carriers and noncarriers. These data continue to inform regulatory strategies in the UK and Europe.
Lecanemab’s therapeutic precision stems from its selective binding to soluble Aβ protofibrils—recognized as early neurotoxic agents in Alzheimer’s—while largely sparing fibrillar amyloid associated with cerebral amyloid angiopathy. This targeting mechanism likely accounts for the therapy’s relatively low rate of ARIA-E, a key safety concern with anti-amyloid treatments.
Real-world US data further addressed implementation challenges, indicating that MRI safety monitoring did not significantly delay initiation and that physician prescribing patterns aligned closely with US FDA guidance. In January 2025, the US FDA accepted Eisai’s Biologics License Application (BLA) for a Subcutaneous (SC) formulation of lecanemab designed for maintenance dosing. This regulatory milestone represents a step forward in expanding access and reducing treatment burden, complementing Eisai’s ongoing rollout of a once-monthly Intravenous (IV) regimen and paving the way for a self-administered option expected by late 2025.
Eisai is also advancing lecanemab into the preclinical Alzheimer’s space, with trials targeting this population anticipated in 2028. From a market perspective, Eisai’s 2024 projections globally estimate approximately USD 2 billion in revenue by 2026, with long-term forecasts reaching more than USD 7 billion by 2032. These numbers reflect the growing confidence in lecanemab’s role as a scalable, precision-based therapeutic anchor in the evolving Alzheimer’s treatment paradigm.
Scaling Up Innovation in Alzheimer’s Disease
AD/PD 2025 marks a critical inflection point in Alzheimer’s disease research, reflecting the field’s transition from symptom-focused care to precision, disease-modifying interventions. This shift is driven by a robust lineup of late-stage Alzheimer’s disease therapeutic candidates—such as LEQEMBI (subcutaneous), valiltramiprosate (ALZ-801), HMTM, and blarcamesine—that target diverse pathological mechanisms, including Aβ, tau aggregation, neuroinflammation, and synaptic dysfunction. These molecules represent the maturation of the Alzheimer’s pipeline and reflect the field’s growing understanding of disease biology. As per DelveInsight’s analysis, each of these drugs—valiltramiprosate (ALZ-801), HMTM, and blarcamesine—is projected to achieve blockbuster status, potentially generating over USD1 billion in revenue by mid-2030, provided they receive regulatory approval.
Lecanemab’s real-world data validate its feasibility and sustained benefit, while the oral agents demonstrate potential for scalability and patient-centered delivery. Notably, these advancements are guided by biomarker-driven strategies and enriched subgroup analyses, especially within genetically defined populations such as APOE4 carriers. Regulatory bodies are responding with expedited pathways, underscoring a paradigm shift toward individualized care. Together, these late-stage developments are reshaping Alzheimer’s treatment into a more stratified, mechanism-based, and accessible framework for long-term disease management.
DevleInsight’s Alzheimer’s Disease Pipeline Analysis
According to DelveInsight’s pipeline team, more than 60 molecules are currently under evaluation across various phases of Alzheimer’s disease clinical trials. Notably, around 25% of these candidates are in Phase III and may reach the market in the near future. The introduction of these therapies is anticipated to substantially enhance the treatment landscape and improve patient outcomes. A detailed comparison of the emerging Alzheimer’s disease drugs is provided below.
The anticipated launch of these emerging therapies for Alzheimer’s disease are poised to transform the market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the Alzheimer’s disease market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.

Downloads
Article in PDF
Recent Articles
- KEY CLINICAL OUTCOMES
- Zealand Pharma’s Phase III Results of Glepaglutide; FDA Approves Amylyx’s ALS Drug Relyvrio; Novo...
- Chimerix Submits Dordaviprone NDA for Accelerated Approval in Recurrent H3 K27M-Mutant Diffuse Gl...
- Rise In Bispecific Antibodies Utilization As Antibody Therapeutics
- Does coconut oil help prevent dementia?



