Hunter Syndrome Market experiences a positive push as the pharma companies spot the untapped opportunities
May 22, 2020
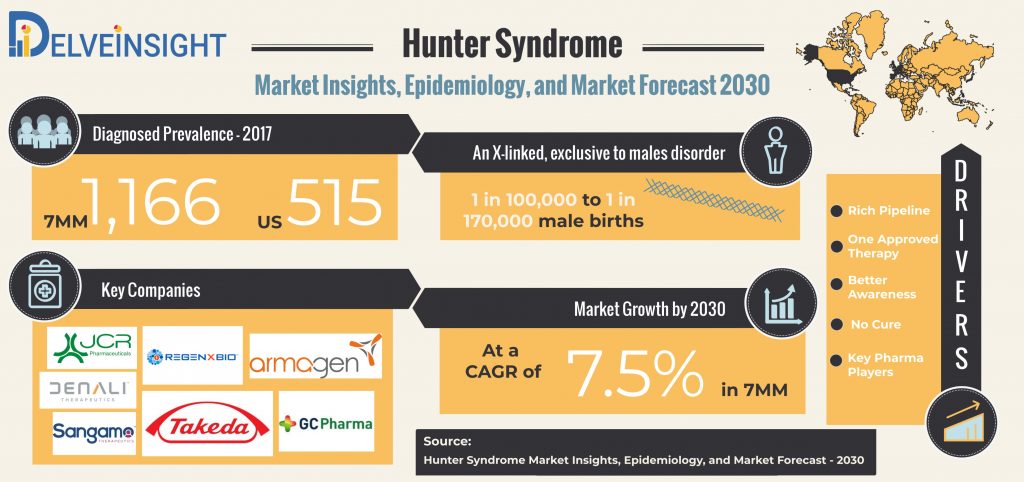
The Hunter Syndrome Market is expected to grow with CAGR of 7.5% in the coming years in the 7MM.
Occurring in approximately 1 in 100,000 to 1 in 170,000 male births, Hunter Syndrome, is an X-linked disorder, of carbohydrate metabolism that occurs almost exclusively in males, with females as the carriers of the diseases. Hunter Syndrome, also known as Mucopolysaccharidosis type II (MPS II), is an Iduronate 2-sulfatase (I2S) deficiency, which is an enzyme responsible for breaking down complex sugar molecules in the body. Deficiency or lack of the enzyme results in an abundance of complex molecules, ultimately leading to progressive and permanent damage to the tissues and organs in the body affecting mental and physical activities as well as appearances.
At birth, it often undergoes undiagnosed as the onset of symptoms does not take place until the age of 4 years. By then, the affected child develops distinctive facial features such as full lips, large rounded cheeks, a broad nose, and an enlarged tongue (macroglossia). Additionally, enlargement of vocal cords, liver and spleen can also be observed, and there is an accumulation of cerebrospinal fluid, a condition known as hydrocephalus. Narrowing of the airway causes frequent upper respiratory infections and short pauses in breathing during sleep (sleep apnea).
Downloads
Article in PDF
Recent Articles
- Decoding Parkinson’s Diagnosis with Gene Therapies and Prevention Insights
- Denali’s Impressive Research Portfolio
- Rising of Orphan Drug Development
- Mucopolysaccharidosis I (MPS I) (Hurler Syndrome) – a lesser developed window
- Mucopolysaccharidosis: How the Different Types Affect Patients and Treatment Approaches?
As the disease progresses, the patient’s mental abilities start to worsen consequently leading to death. It is grim to note that the life expectancy of these individuals is 10 to 20 years. In the mild form of the disease, average life expectancy is about 20 years.
According to DelveInsight assessments, total diagnosed Hunter Syndrome prevalence in the 7MM was observed to be 1,166, and for the US, the number was approximately 515. Although rare, awareness of Hunter Syndrome is warranted among the physicians; however, there is no approved-cure that can kill the disease from the root. The treatments for rare diseases and associated costs pose as significant hurdles in the quest of reducing the healthcare burden the condition poises globally.
The Hunter Syndrome treatment market is multi-disciplinary, and there exist treatment regimens that are symptomatic. The multi-disciplinary approach to manage Hunter Syndrome, in a way, becomes imperative due to the progressive nature of the disease and significant clinical heterogeneity.
The major treatment option in Hunter Syndrome Market is Enzyme Replacement Therapy (ERT). The therapy is available for several other lysosomal disorders as well; however, it helps in an increase in the concentration of deficient enzyme, ameliorating the somatic features of the disorder and does not rectify the genetic error. Idursulfase (Elaprase) developed by TKT Therapeutics, now sponsored by Takeda, is an FDA-approved ERT for Hunter syndrome and has shown to improve the walking abilities of the patients. However, the therapy is suitable for advanced or severe cases of Hunter Syndrome, and also has its clinical limitations in the form of inability to cross the blood-brain barrier (BBB), thus unable to alleviate neurological symptoms. Initial or mild cases are directed for surgery, developmental, occupational, and physical therapy are all symptomatic in nature and does not treat the disorder.
With the help of surgeries, hernia and joint contractures can be corrected, or surgical removal of tonsils (Tonsillectomy) and the adenoids or both (adenoidectomy) can help with breathing-related issues, and or to treat possible hydrocephalus. Moreover, to keep the breathing passage working and open, positive pressure ventilation (CPAP or tracheostomy) is used, then there are hearing devices to treat hearing loss. Developmental, physical, and occupational therapy can be helpful. Genetic counselling is recommended for families that have a child with MPS II. Moreover, hematopoietic stem cell transplantation is also an alternative; however, there were limited clinical benefits in patients with this disease and has been associated with a severe risk of morbidity and mortality.
Hunter Syndrome Market
It is not wrong to say that the present Hunter Syndrome Market has several unmet needs in the form proper diagnosing of the disease as it often gets misdiagnosed as Mucolipidosis II, Mucolipidosis III Alpha/Beta, and Mucolipidosis III Gamma, and other lysosomal storage disorders. Moreover, it gets diagnosed properly; it often results in compromised quality of lives of the patients as well as the care takes as along with the varying treatment regimens the treatment also adds the burden of cost. It is not to be missed that tracking the course of the disease is also a considerable challenge in itself as the severity defines the treatment modalities that are to administer.
However, to overcome these challenges, researchers have shifted their focus to the development of well-tolerated therapies that can cross the blood-brain barrier. For instance, an approach which involves the infusion of ERT into the cerebrospinal fluid is under evaluation that is thought to help in enabling widespread distribution of the enzyme throughout the CNS. Other areas of research include the use of pharmacological chaperones, gene therapy, and substrate reduction therapy.
Moreover, DelveInsight estimated that looking at the present Hunter Syndrome market scenario, upcoming therapies, and rising awareness, the Hunter Syndrome Market is expected to experience a positive push in the coming years with a CAGR of 7.5% for 7MM. The lack of therapies to effectively stop or slow the disease progression offers a potential opportunity for the development and launch of novel therapies. More or less, the future of the Hunter Syndrome market looks bright with several pharma companies developing novel therapies in the Hunter Syndrome Market with Takeda expected to occupy the major share of the market. Additionally, other emerging players accelerating Hunter Syndrome Pipeline include JCR Pharmaceuticals (JR-141), Green Cross Corporation (GC1111B or Hunterase), Regenxbio Inc. (RGX-121), GC Pharma, Denali Therapeutics (DNL310 ), Sangamo Therapeutics (SB-913), and ArmaGen have recently shifted their focus in advancing Hunter Syndrome Market with several of the novel therapies in the pipeline that promise to bring relief to the males with Hunter Syndrome.
Downloads
Article in PDF
Recent Articles
- Rising of Orphan Drug Development
- Overcoming the prevailing unmet needs in Beta-Thalassemia Market
- Decoding Parkinson’s Diagnosis with Gene Therapies and Prevention Insights
- Mucopolysaccharidosis: How the Different Types Affect Patients and Treatment Approaches?
- Mucopolysaccharidosis I (MPS I) (Hurler Syndrome) – a lesser developed window