AAV Gene Therapies for Hemophilia B Treatment: The Road to a Cure
Oct 27, 2023
Table of Contents
Hemophilia B is a rare genetic bleeding disorder in which affected individuals have insufficient levels of a blood protein called factor IX. Around 3 in 100 individuals with hemophilia B produce an antibody to the factor IX replacement therapy used to treat or avoid their bleeding episodes, called an inhibitor. The inhibitor prevents the therapy from working, which makes it harder to avoid an episode of bleeding.
As per DelveInsight’s analysis, in 2022, the total treated prevalent population of hemophilia B in the US was approximately 4K. As per the estimates it can be observed that moderate hemophilia B cases are higher in number than mild and severe cases in the US.
Downloads
Article in PDF
Recent Articles
- Gene Therapy in Rare Disorders: Acceptance in Europe Faces Challenges
- Aelis Farma pockets $30M; Alcyone unveils $23M for AAV gene therapies; Progentec and GSK collabor...
- Role of Mobile Technology in Hemophilia Management
- Gilead Buys Out Rights to Cancer Therapy from Jounce; FDA Places Clinical Hold on Biogen’s Orelab...
- Gene Therapy: The Next Milestone in Treating Complex Diseases
Hemophilia B is a life-long condition. Currently, there is no cure, but researchers are actively engaged in finding the cure through gene therapy. One hope is that by inserting a healthy version of the defective blood factor gene, a person with hemophilia will be able to produce reasonable amounts of a factor on their own.

HEMGENIX: The Only Approved Gene Therapy for Hemophilia B Treatment
Gene therapy offers an exciting avenue for achieving a long-lasting cure in a single treatment for X-linked bleeding condition hemophilia, as opposed to the various ongoing clinical treatments. Patient data corroborates the potential for sustained, natural clotting factor production through gene therapy, eliminating the need for frequent injections of coagulation factors or alternative drugs. This has the potential to significantly reduce the overall cost of care. Hemophilia B stands out as an ideal candidate for gene therapy due to its varying factor level sensitivity to bleeding symptoms and the non-essential need for precise control. Moreover, only a fraction of hepatocytes need correction to effectively address the bleeding diathesis, as clotting factor proteins are secreted into the bloodstream.
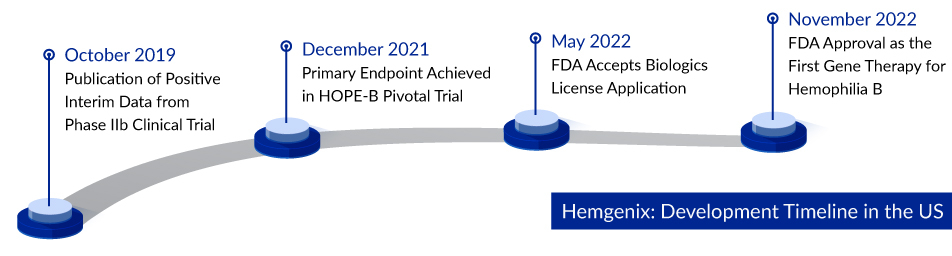
In a milestone achievement, HEMGENIX (CSL Behring/uniQure), the groundbreaking gene therapy approved in 2022, has received the green light for treating adults grappling with hemophilia B, a genetic disorder marked by a shortage of Factor IX. Designed for those who currently rely on Factor IX prophylaxis, have survived life-threatening hemorrhages, or frequently experience severe spontaneous bleeding incidents, HEMGENIX exhibited remarkable results in the Phase III clinical trial known as HOPE-B. This cutting-edge therapy demonstrated an impressive 64% reduction in all bleeding incidents and an astounding 77% decrease in bleeding episodes necessitating Factor IX treatment over a span of seven to eighteen months. Remarkably, the price tag for HEMGENIX is a staggering USD 3.5 million per dose, solidifying its status as the world’s most expensive medication and a groundbreaking milestone as the first gene therapy approved for this rare disease.
Promising Gene Therapies in Pipeline for Hemophilia B Treatment
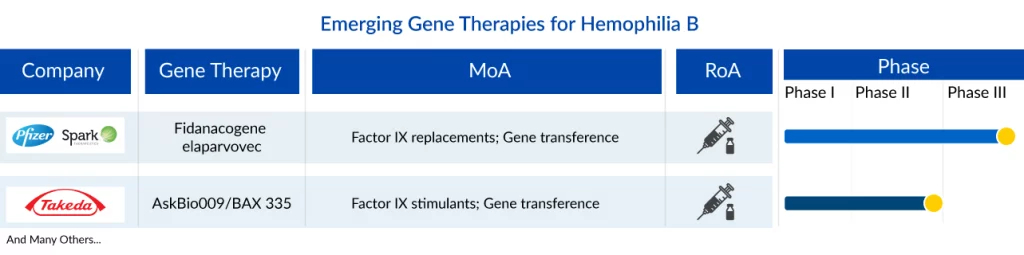
The landscape of the hemophilia B treatment market is poised to undergo significant changes in the near future due to the increasing global healthcare expenditure. Leading pharmaceutical companies such as Pfizer/Spark Therapeutics (SPK-9001), Takeda (AskBio009), and others are dedicated to advancing new therapies for hemophilia B, aiming to enhance symptom management and ultimately elevate the quality of life for patients.
Fidanacogene elaparvovec, previously SPK-9001 or PF-06838435 is a novel, investigational bio-engineered AAV vector by Pfizer/Spark Therapeutics which utilizes a high-activity F9 transgene for hemophilia B or factor IX deficiency. Currently, SPK-9001 is under Phase III (NCT03861273) clinical trial study. In September 2015, SPK-9001 received an orphan drug designation from the US FDA, and later in July 2016, it received breakthrough therapy designation from the FDA and PRIME from the EU. Pfizer recently announced that the US FDA has accepted a BLA for fidanacogene elaparvovec to treat adults with hemophilia B. The EMA has also accepted a marketing authorization application currently under review. This submission was based on the findings from the ongoing Phase III BENEGENE-2 study (NCT03861273). It enrolled 45 participants who had completed at least six months of routine factor IX (FIX) prophylaxis therapy in the lead-in study (NCT03587116) and received a dose of fidanacogene elaparvovec.
AskBio009/BAX 335 is another gene therapy in the early stage of development for hemophilia B treatment. BAX 335 is an adeno-associated virus serotype 8 (AAV8)-based clotting factor IX (FIX) Padua (R338L) gene therapy for the treatment of hemophilia B. Takeda is currently conducting Phase I/II trial for AskBio009. As per Phase I/II results, no serious adverse event occurred. No clinical thrombosis, inhibitors, or other FIX Padua-directed immunity was reported. FIX expression was measurable in 7 of 8 participants; peak FIX activity displayed dose dependence (32.0% to 58.5% in cohort 3).
Many gene therapies for hemophilia B treatment have been stopped in their development stages, such as Verbrinacogene setparvovec (FLT 180a) (Freeline Therapeutics) and SB-FIX (Sangamo Therapeutics). The field of gene therapy for hemophilia B treatment has a low success rate and few key players. Most of the key players are developing their gene therapies outside the 7MM, mainly in China.
Challenges with Gene Therapies for Hemophilia B Treatment
Despite the widespread availability of safe and effective replacement therapy, patients with hemophilia B continue to bear a substantial treatment burden, including issues like breakthrough bleeding, progressive joint disease, and the development of inhibitors. This unmet need is particularly pressing, given that hemophilia B affects approximately 3 in 100 people with hemophilia B, leading to increased treatment costs and greater risks to patient well-being. The emergence of inhibitors further compounds the problem by reducing the efficacy of factor IX in blood coagulation, negatively impacting patients’ health, quality of life, and significantly driving up the overall cost of hemophilia B treatment.
Moreover, the diagnosis of hemophilia B often faces delays, primarily due to factors like a clinical presentation that mirrors other hemorrhagic disorders and the delayed onset of symptoms. The predominant treatment options typically involve intravenous administration. Many patients, particularly children, express discomfort and inconvenience with this method. Treating hemophilia B comes at a considerable cost, and future treatment options, such as gene therapies and extended half-life products, are also anticipated to be on the expensive side. Health authorities are likely to implement measures to control the pricing and utilization of these high-cost therapies.
The Bright Future of Gene Therapies for Hemophilia B Treatment
Currently, non-inhibitor drug candidates are the driving force behind hemophilia B treatment. The emergence of promising new contenders represents a notable disruption to the hemophilia B pharmaceutical industry, as they have the potential to reshape the standard of care for patients. The landscape of hemophilia B treatment is continually shifting, with numerous companies diligently pursuing the development of novel therapies that could bring transformative changes to how we treat hemophilia B.

As per DelveInsight analysis, by 2025, the total market size of hemophilia B AAV gene therapies in the 7MM is estimated to be approximately ~300 USD million. The current standard of care treatment for severe hemophilia B is the prophylactic intravenous replacement of coagulation factor IX (FIX) to prevent spontaneous bleeding.
Currently, HEMGENIX holds the exclusive approval as an AAV gene therapy for hemophilia B. Nevertheless, a new contender, fidanacogene elaparvovec, is gaining momentum in the development hemophilia B pipeline. Both treatments function by delivering a functional variant of the mutated factor IX gene, intensifying the rivalry between these two therapies, each vying to rectify the fundamental genetic defect and provide a cure for hemophilia B.
In conclusion, the future of gene therapies for hemophilia B treatment holds great promise, offering the potential for a paradigm shift in treatment approaches. As gene therapy continues to advance, addressing challenges and refining techniques, it has the potential to significantly improve the lives of individuals living with hemophilia B. While there is still much work to be done, the journey towards more effective and accessible treatment options is well underway.

Downloads
Article in PDF
Recent Articles
- Ocugen-Bharat Biotech’s Covaxin Against B.1.617 Corona Variant; uniQure Shares; J&J Covid Va...
- Sanofi’s Qfitlia Enters the Hemophilia Market—What Sets It Apart?
- Actinium Announces SIERRA Trial Results; Santhera Seeks FDA Review for Vamorolone; Seres Announce...
- Plasma Proteins Therapeutics: Transforming Treatment in Hematology and Beyond
- Gene Therapy: The Next Milestone in Treating Complex Diseases