Pfizer’s ABRYSVO Outpaces GSK’s AREXVY with Expanded FDA Approval – But Can It Sustain the Momentum?
Oct 28, 2024
Pfizer’s ABRYSVO became the first and only RSV vaccine approved for use in adults under 50, offering the most extensive RSV vaccine coverage for adults. The label expansion announced on 22 October 2024 includes younger adults considered at higher risk for RSV-related lower respiratory tract disease (LRTD).
ABRYSVO (RSVpreF) is a sterile solution for intramuscular injection. The vaccine is supplied as a vial of lyophilized antigen component that is reconstituted at the time of use with a sterile water diluent component. The antigen component contains recombinant RSV preF A and RSV preF B.
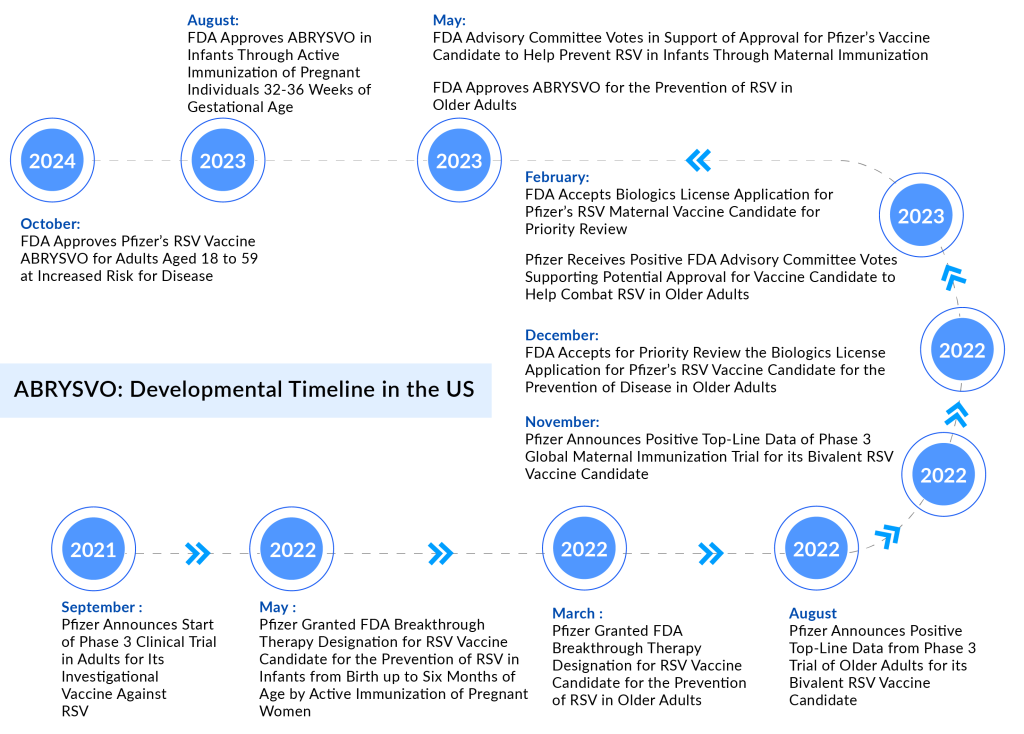
In May 2023, the FDA approved ABRYSVO to prevent LRTD caused by RSV in individuals aged 60 and older. In August 2023, the FDA further approved ABRYSVO for the prevention of LRTD and severe LRTD due to RSV in infants from birth to 6 months of age through the active immunization of pregnant individuals between 32 and 36 weeks of gestation.
Downloads
Article in PDF
This was followed by a recommendation from the Advisory Committee on Immunization Practices (ACIP) in September 2023, advocating for maternal immunization to protect newborns during the RSV season, suggesting the administration of the vaccine from September to January in most of the continental United States.
In August 2023, Pfizer also announced that the European Commission had granted marketing authorization for ABRYSVO for use in older adults and for maternal immunization to protect infants. Moreover, ABRYSVO has received approvals for both indications in various countries around the world.
While Pfizer’s ABRYSVO is a win against GSK’s RSV vaccine, AREXVY, which is approved for individuals aged 50 and older, it’s unclear how much benefit will come from this approval since the Centers for Disease Control and Prevention (CDC) still needs to authorize ABRYSVO for a broader age group.
Get more insights on GSK’s RSV vaccine AREXVY
In June 2024, the CDC limited its recommendation for RSV vaccines to adults aged 75 and older, as well as those between 60 and 74 who are at risk of severe illness. Previously, the CDC had recommended the vaccines for all adults aged 60 and older.
The FDA’s decision is based on inferred efficacy from the pivotal Phase III clinical trial (NCT05842967) known as MONeT (RSV Immunization Study for Adults at Higher Risk of Severe Illness). This trial evaluated the safety, tolerability, and immunogenicity of ABRYSVO in adults who are at risk of RSV-related disease due to specific chronic medical conditions. The company plans to submit the MONeT results for publication in a peer-reviewed scientific journal and to present them at an upcoming scientific conference.
In the US, 9.5% of adults aged 18 to 49 have an underlying chronic condition, such as obesity, diabetes, chronic obstructive pulmonary disease (COPD), heart failure, chronic kidney disease, or asthma, which increases their risk of developing and being hospitalized for RSV-associated LRTD. This percentage rises to 24.3% among individuals aged 50 to 64.
Last year, ABRYSVO brought in $890 million in sales, while GSK announced that AREXVY’s revenue hit 1.2 billion pounds sterling ($1.5 billion). Following the CDC’s revised recommendation, GSK lowered its 2024 sales forecast from a high single-digit to low double-digit percent growth to a low to mid-single-digit percent growth at constant exchange rates.
Alongside ABRYSVO and AREXVY, a new player has entered the RSV adult market, i.e., Moderna’s mRESVIA. In May 2024, Moderna’s mRESVIA received FDA approval to safeguard adults aged 60 and older from lower respiratory tract disease caused by respiratory syncytial virus. This marks Moderna’s second FDA approval, following the 2022 authorization of its COVID-19 vaccine, Spikevax. Similar to Spikevax, mRESVIA utilizes Moderna’s messenger RNA technology.
Sanofi and AstraZeneca are also capturing the RSV vaccine market with their product BEYFORTUS. In July 2023, the FDA approved Sanofi and AstraZeneca’s antibody, BEYFORTUS (nirsevimab), as a preventive treatment for RSV lower respiratory tract disease in infants. This approval applies to newborns and infants who are either born during or entering their first RSV season, as well as to young children up to 24 months old who are still at risk for RSV during their second season of illness.
Dive deep to get the complete story of the approval of Sanofi/AstraZeneca’s BEYFORTUS for infants
Talking about AstraZeneca, it is also coming up with another product IVX-A12. In February 2024, AstraZeneca completed the acquisition of ICOSAVAX. The proposed acquisition focused on AstraZeneca’s expertise in RSV, strengthening AstraZeneca’s Vaccines & Immune Therapies late-stage pipeline with ICOSAVAX’s lead investigational vaccine candidate, IVX-A12.
IVX-A12 is a potential first-in-class combination RSV vaccine candidate containing VLPs that incorporate stabilized prefusion F proteins from RSV and hMPV viruses. The FDA has granted IVX-A12 Fast Track designation to adults ≥60 years of age.
Another contender in the race is Blue Lake Biotechnology’s BLB201. BLB-201 is being developed to prevent RSV infections. This therapeutic candidate utilizes Parainfluenza virus 5 (PIV5, CPI strain) to develop amplifying virus-like particles that express RSV antigens and is administered intranasally. The vaccine is based on the PIV5 platform.
In March 2024, Blue Lake Biotechnology announced promising preliminary results from the first two cohorts of a Phase I/IIa trial of BLB-201, an investigational RSV vaccine. The vaccine demonstrated immunogenicity and was well-tolerated in children.
Meissa is advancing MV-012-968, a live-attenuated, needle-free intranasal vaccine candidate designed to protect infants from RSV. In Phase I clinical trials, this RSV vaccine was well-tolerated, significantly attenuated, and elicited a robust RSV-specific mucosal IgA response in participants (both adults and young children) who had prior RSV infections and circulating antibodies in their blood.
Learn more about the evolving RSV treatment landscape at Respiratory Syncytial Virus Treatment Market: A Complex Space Worth Billions!
The launch of these emerging RSV vaccines is set to revolutionize the market in the coming years. However, there remains an unmet need for effective, safe, and affordable preventive and therapeutic options for RSV, including antiviral treatments and extended half-life monoclonal antibodies. Developing safe and effective RSV vaccines or monoclonal antibodies faces significant challenges, especially regarding rigorous safety standards for pediatric and maternal populations. New RSV vaccines could potentially reduce the global burden of this virus.

Downloads
Article in PDF
Recent Articles
- Aurinia’s Lupkynis for Lupus; FDA Fast Track Designation for Toripalimab; Wren Therapeutics...
- Alzheimer’s drug fails! Is it time to move on?
- Sinusitis: Something More Troublesome Than Just Cold
- Notizia
- FDA approval to AZ’s Farxiga for heart failure; GSK divests two travel vaccines to Bavarian Nord...