Understanding the Acute Myeloid Leukemia Surge and the Road to Solutions
Jan 22, 2025
Table of Contents
Acute Myeloid Leukemia (AML) represents a significant public health concern globally, with its prevalence and incidence on the rise. According to the American Cancer Society’s estimates for 2025, approximately 22K people in the United States are expected to be diagnosed with AML, with most cases occurring in adults. The disease is notably severe, as around 11K individuals are projected to die from AML in the same year, again predominantly affecting adults. While AML accounts for about 1 out of 3 leukemias in adults, it remains relatively uncommon overall, constituting about 1% of all cancers. The average age at diagnosis is approximately 69 years, and while AML can occur in children, it is rare in those under 45.
DelveInsight’s analysis further emphasizes the increasing incidence of AML across the 7MM. In 2023, the total number of incident cases of AML was around 43.5K, with the United States reporting the highest number. Among the EU4 countries (Germany, France, Italy, and Spain) and the UK, Germany had the highest incidence rates of AML, while Spain recorded the lowest. The disease shows a slight male predominance.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- DelveInsight launches Indication Active Pharmaceutical Ingredient (API) Reports
- Pancreatic Cancer-Pipeline Insights, 2016
- Antibody-Drug Conjugate Market Insight
- Non-Surgical Body Contouring: What’s Fueling Its Growth in Aesthetic Medicine?
- How the Amniotic Membrane Market is Transforming Regenerative Healthcare Worldwide
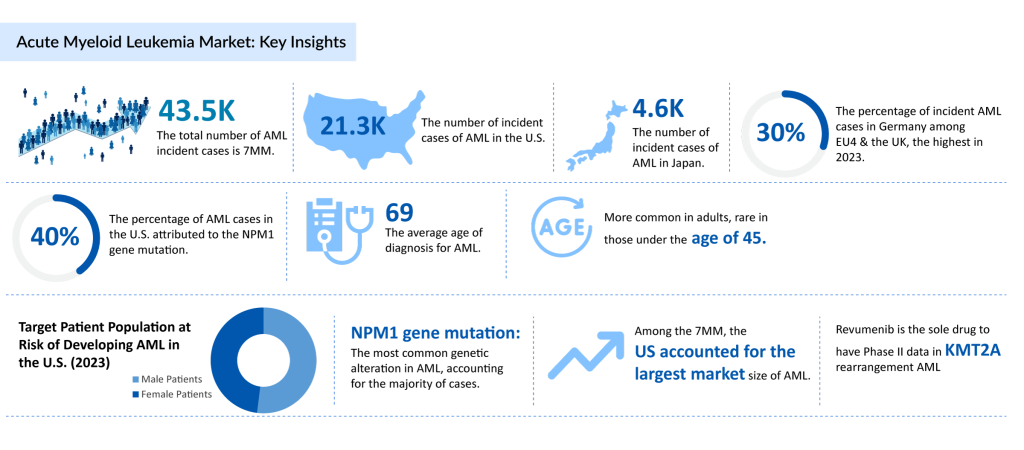
The genetic landscape of AML also plays a crucial role in its spread. A significant portion of AML patients carry the NPM1 gene mutation, which is one of the most common genetic abnormalities associated with the disease. Understanding these genetic factors is essential for developing targeted therapies that can improve treatment outcomes.
As the burden of AML continues to grow, these statistics highlight the urgent need for enhanced research efforts and healthcare strategies aimed at prevention, early diagnosis, and effective treatment of this challenging disease.
What are the Factors Driving Incidence of AML?
Several key factors influence the incidence of Acute Myeloid Leukemia (AML), underscoring the complexity of its onset and progression. One of the most prominent risk factors is myelodysplastic syndrome (MDS), a group of disorders caused by poorly formed or dysfunctional blood cells, which significantly raises the likelihood of AML development. Additionally, hematological conditions like myelofibrosis and aplastic anemia further elevate the risk of AML by disrupting normal blood cell production.
Acute myeloid leukemia symptoms often emerge rapidly, as the disease progresses quickly, leading to a decline in normal blood cell counts. Common symptoms include fatigue, frequent infections, and easy bruising or bleeding, which typically develop over a short period, often just days or weeks, before diagnosis. The final stages of acute myeloid leukemia can be particularly challenging, as the disease may cause severe complications like organ failure or critical infections, highlighting its aggressive nature.
When considering how deadly is acute myeloid leukemia, its prognosis varies based on factors such as age, overall health, and specific genetic mutations. Although rare, acute myeloid leukemia in children is also a concern, and while treatment outcomes in pediatric cases are generally better than in older adults, early detection and intervention remain critical. Environmental factors, such as previous chemotherapy or radiation exposure, and genetic mutations further contribute to AML’s rising incidence, emphasizing the need for vigilant monitoring and early diagnosis in at-risk populations.
Economic Burden of Acute Myeloid Leukemia
The economic burden of Acute Myeloid Leukemia is significant, especially for younger patients and those requiring high-intensity treatments. Treatment costs vary based on factors like treatment type, location, and the patient’s specific condition. High-intensity therapies such as induction chemotherapy, hematopoietic stem cell transplantation, and the management of relapsed or refractory cases represent the most expensive treatment options. The most significant cost driver across all treatment episodes is inpatient hospitalization, as extended hospital stays for intensive treatments and supportive care can quickly escalate costs. Additionally, reimbursement rates for medical claims vary depending on the insurance plan, further influencing the financial burden on patients and healthcare systems.
Although treatment costs are well-documented, limited evidence exists regarding the ongoing economic burden after achieving remission. Little is known about how costs differ for older patients treated with standard intensive chemotherapy compared to those using less intensive hypomethylating agent (HMA) regimens, which are commonly used in older populations. A recent US-based study aimed to address this gap by assessing treatment patterns and clinical outcomes, including overall survival and relapse-free survival, in patients aged 65 and older who achieved remission. Understanding the financial implications of various treatment regimens, particularly for older adults, is crucial to shaping future treatment strategies and mitigating the long-term economic burden of AML.
AML Market Scenario and Market Size
The market size for Acute Myeloid Leukemia remains undefined due to no approved targeted therapies. However, among the 7MM, the United States has consistently accounted for the largest market share. In Europe, Germany led the market in 2023 among the EU4 (Germany, France, Italy, and Spain) and the United Kingdom, while Spain represented the smallest market size in the region.

A significant unmet need exists in the treatment of the NPM1-mutant AML subgroup, which makes up approximately 30% of new AML cases annually. The lack of approved targeted therapies for this subgroup emphasizes the critical need for innovation. Ziftomenib, which became the first investigational treatment to receive Breakthrough Therapy designation for NPM1-mutant AML in April 2024, represents a significant step forward. Furthermore, Syndax Pharmaceuticals is advancing the clinical development of Revumenib, a treatment aimed at acute leukemias with mutant NPM1 and KMT2A rearrangements. Revumenib’s ongoing trials, in combination with standard-of-care (SoC) therapies, could expand its use across multiple treatment settings, including frontline, relapse, refractory, and post-transplant maintenance, offering hope for addressing the unmet needs in AML treatment.
Market Dynamics of AML
The primary goal of treatment for Acute Myeloid Leukemia is remission, with conventional therapies typically involving intensive chemotherapy-based induction and consolidation treatments, along with hematopoietic stem cell transplantation. Healthcare providers often reserve these therapies for younger, healthier patients because they are physically and mentally demanding. The standard regimen for AML treatment has long been a combination of cytarabine and an anthracycline, such as daunorubicin, which continues to be the backbone of treatment for fit patients. For those in remission, allogeneic stem cell transplantation offers a potential cure. However, this approach is not suitable for all patients, particularly those who cannot tolerate the intensity of chemotherapy.
Key Players and Drugs in AML Treatment Landscape
In response to the limitations of traditional treatments, targeted therapies have emerged as a crucial component of AML management. Drugs like RYDAPT and XOSPATA target FLT3 mutations, which are found in about 30% of AML patients, while TIBSOVO and IDHIFA address specific IDH1 and IDH2 mutations, respectively. Additionally, VENCLEXTA, a BCL-2 inhibitor, is often used in combination with hypomethylating agents or low-dose cytarabine for patients who are not candidates for intensive chemotherapy. These treatments are helping to address the unmet needs of patients who are ineligible for traditional therapies.
The market for AML therapies is expanding rapidly as new, innovative treatments enter the scene. Research is actively exploring targeted therapies, immunotherapies, and cell-based treatments such as CAR-T cell therapies, monoclonal antibodies, and checkpoint inhibitors. These new therapeutic options hold the potential to significantly improve outcomes for AML patients. The AML treatment market is expected to grow significantly, driven by pharmaceutical companies such as Daiichi Sankyo, Agios Pharma, Astellas Pharma, Rigel Pharmaceuticals, Jazz Pharmaceuticals, Novartis, Pfizer, Abbvie, BMS, Arog Pharmaceuticals, Actinium Pharmaceutical, Astex Pharmaceutical, Syndax, Geron, SELLAS Life Sciences, Johnson & Johnson Innovative Medicine, Sanofi, which are at the forefront of developing and marketing new therapies.
Speedbumps in the Race for AML Treatment Innovation
The market for treating Acute Myeloid Leukemia (AML) faces several significant barriers, primarily revolving around cost and access. The development, approval, and commercialization of new therapies are often expensive, resulting in high treatment costs that burden both patients and healthcare systems. Conventional therapies, such as chemotherapy and stem cell transplantation, also carry substantial expenses due to hospital-based treatments, frequent monitoring, and managing side effects. Low- and middle-income countries face a pronounced financial burden due to limited access to the latest treatments and comprehensive supportive care. Additionally, individuals without adequate health insurance may face significant out-of-pocket expenses, further restricting access to essential care for high-risk AML leukemia patients.
In addition to cost, the side effects associated with conventional and newer therapies add complexity to the AML treatment landscape. Chemotherapy often causes severe side effects, including fatigue, nausea, and an increased risk of infections, which can significantly impact a patient’s quality of life and require additional care. While newer targeted therapies offer reduced toxicity, they can still produce side effects requiring careful management. The prognosis for AML remains challenging, particularly for older patients or those with comorbidities. Despite advancements, the acute myeloid leukemia survival rate varies widely based on age, genetic mutations, and the specific characteristics of the disease, with lower rates seen in high-risk subtypes.
Other challenges, such as delayed diagnosis and limitations in designing effective clinical trials, further hinder progress. Combined with the historically low survival rate for AML patients, these barriers highlight the need for innovations that can improve treatment strategies, reduce financial strain, and ensure equitable access to care for all AML patients.
Conclusion
The Acute Myeloid Leukemia therapeutic market is on the verge of transformative advancements, driven by significant progress in acute myeloid leukemia treatments and the development of innovative therapeutic modalities. Emerging therapies such as Ziftomenib and Revumenib, along with CAR-T cell therapies, monoclonal antibodies, and immunotherapies, are reshaping the treatment paradigm. These advancements are particularly impactful for subgroups like NPM1-mutant and FLT3-mutant AML patients, offering personalized approaches that enhance survival outcomes and alter the acute myeloid leukemia prognosis.
As the understanding of Acute Myeloid Leukemia deepens, efforts are accelerating to address critical needs, including improving access to advanced therapies in underserved regions. Focusing on early diagnosis and comprehensive care strategies is transforming patient treatment outcomes, including for those in the final stages of acute myeloid leukemia. Collaborative initiatives among pharmaceutical companies, healthcare providers, and policymakers will ensure these therapies reach a broader patient base.
The advancements in acute myeloid leukemia treatments bring renewed hope, extending life expectancy and enhancing the quality of life across all stages of the disease. These innovations mark a significant leap forward, offering patients and their families a brighter outlook in the battle against AML.