All Blogs
May 22, 2023
Chondrosarcoma Treatment Market: Insight on Patient Burden and Inhibrx’s INBX-109 US Market Entry in 2026
Chondrosarcoma is a group of bone tumors made up of cells that make too much cartilage. As per DelveInsight analysis, the total incident cases of chondrosarcoma in the 7MM comprised approximately 2,331 cases in 2022 and are projected to increase during the forecasted period. Our estimates suggest that the United St...
Read More...
May 19, 2023
Graves’ Ophthalmopathy Treatment Market: A Billion-Dollar Opportunity For Pharma Companies
Graves’ ophthalmopathy, also known as thyroid-associated ophthalmopathy and thyroid eye disease, is the most frequent extrathyroidal manifestation of Graves’ disease. The majority of Graves’ ophthalmopathy cases are caused due to Graves’ disease, and only a small percentage of these patients arise from other euthyr...
Read More...
May 18, 2023
FDA Approves Abbott’s Spinal Cord Stimulation Systems; Regen Lab Received CE Certification for Three PRP Solutions; Beckman Coulter Launched New Immunoassay Analyser; Shockwave Medical’s Coronary IVL Catheter; Providence Completed Enrollment in the FUSE Clinical Study; Onward Completed First-In-Human Use of Movement-Restoring Lead
Providence Medical Technology Completed Enrolment in the FUSE Clinical Study for High-Risk Cervical Fusion Patients On May 11, 2023, Providence Medical Technology, Inc. (PMT), a leader in medical device innovation for spine surgery, closed the enrolment in its FUSE Study which is a prospective, multicenter...
Read More...
May 17, 2023
Top Artificial Intelligence-Based Healthcare Mobile Apps and Their Use Cases
The role and application of Artificial Intelligence have grown at an immense pace in recent years. Globally, several companies including large & small scale businesses, education, retail, and telecommunications, among others are actively exploring opportunities to leverage the potential of AI in their organizat...
Read More...
May 16, 2023
Sarepta Therapeutics’s SRP-9001 Gene Therapy; FDA Approves Astellas’ VEOZAH; FDA Orphan Drug Designation and Rare Pediatric Disease Designation to SiSaf’s siRNA Therapy SIS-101-ADO; FDA Grants Fast Track Designation to IMPT-314; FDA Approves First Drug for Agitation in People With Alzheimer’s Disease; FDA Accepted the CytoAgents’ IND Application for CTO1681
Sarepta Therapeutics Announces Positive Vote from U.S. FDA Advisory Committee Meeting for SRP-9001 Gene Therapy Sarepta Therapeutics, Inc., a pioneer in precision genetic medicine for rare diseases, announced that the FDA's Cellular, Tissue, and Gene Therapies Advisory Committee (CTGTAC) voted 8 to 6 in favor of...
Read More...
May 15, 2023
How Decisive Will The OTC Approval of NARCAN Be In The Fight Against The Opioid Epidemic?
On March 29, 2023, the FDA made the landmark decision to approve Emergent BioSolutions’ NARCAN nasal spray for over-the-counter (OTC) and nonprescription use, making it the first naloxone product to be approved for use without a prescription. With about 80,000 fatal opioid overdoses every year, this could potent...
Read More...
May 11, 2023
Norlase’s ECHO™ Green Pattern Laser; HAPPE Spine’sINTEGRATE-C™ Interbody Fusion System; Soterix Medical’s Parcel-Guided rTMS Depression Trial; Akura Medical’s High-Performance Mechanical Thrombectomy Platform; Intelivation Technologies’s Golden Isles™ Minimally Invasive Pedicle Screw System; Signifier Medical Technologies Announced Partnership with Sunrise
Norlase Received FDA 510(k) Clearance and CE Mark Approval For ECHO™ Green Pattern Laser On May 5, 2023, Norlase, a leading global ophthalmic laser manufacturer developing next-generation laser solutions, received both 510(k) US Food and Drug Administration clearance and CE Mark approval for the ECHO Gree...
Read More...
May 12, 2023
Gene Therapy in Rare Disorders: Acceptance in Europe Faces Challenges
By bringing life-changing benefits to people with rare diseases and cancers, cell and gene therapies are reshaping the field of medicine. These treatments have recently gained tremendous publicity for meeting long-standing unmet needs and being extremely costly. But high production costs are limiting patient access...
Read More...
May 10, 2023
How Augmented and Virtual Reality Are Transforming the Healthcare Market Dynamics?
The implementation and adoption of new-age technologies such as Augmented and Virtual Reality (AR and VR) are immensely driving and shaping the future of the healthcare industry with new and innovative solutions. Augmented and Virtual Reality are not new concepts, but tremendous advances have been registered in rec...
Read More...
May 09, 2023
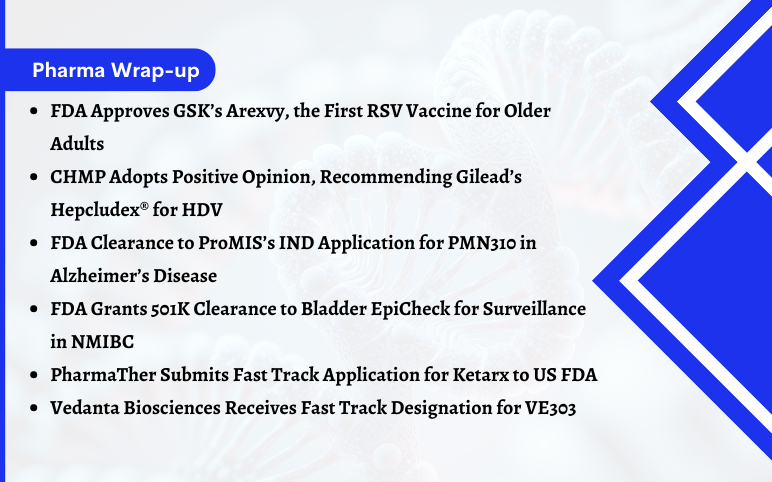
FDA Approves GSK’s Arexvy for RSV; CHMP’s Opinion on Gilead’s Hepcludex® for HDV; FDA Clearance to ProMIS’s IND Application for PMN310; FDA Grants 501K Clearance to Bladder EpiCheck; PharmaTher Submits Fast Track Application for Ketarx to US FDA; Fast Track Designation to Vedanta Biosciences’ VE303
FDA Approves GSK’s Arexvy, the First RSV Vaccine for Older Adults GSK plc stated that the US Food and Drug Administration (FDA) has approved Arexvy (respiratory syncytial virus vaccine, adjuvanted) for the prevention of lower respiratory tract disease (LRTD) caused by a respiratory syncytial virus (RSV) in peopl...
Read More...