ALX Oncology’s Evorpacept Combo Shows Strong Response in Relapsed B-NHL Patients: AACR 2024
Apr 10, 2024
The presentation by ALX Oncology at the AACR Annual Meeting 2024 provided promising insights into the efficacy and safety of their investigational drug, evorpacept, in combination with standard rituximab and lenalidomide for patients with relapsed or refractory B cell non-Hodgkin lymphoma (R/R B-NHL).
The Phase I/II trial included 20 patients with both indolent and aggressive forms of the disease. The data revealed a best overall response rate of 94%, indicating a high level of effectiveness in treating these challenging forms of lymphoma. Impressively, the complete response rate stood at 83%, highlighting the potential of the combination therapy to induce significant tumor regression. Furthermore, the median duration of response was not reached.
One of the most notable aspects of the presentation was the favorable safety profile of the combination regimen. ALX reported that the treatment was well tolerated, with no dose-limiting toxicities observed. Equally important, there were no treatment-related deaths recorded during the study, underscoring the safety of the treatment approach.
Downloads
Article in PDF
Recent Articles
- Clinical activity of P-BCMA-ALLO1, a B-cell maturation antigen (BCMA) targeted allogeneic chimeri...
- First-in-human dose-expansion study of NBF-006, a novel investigational siRNA targeting GSTP, in ...
- Phase I/II trial of copanlisib in combination with nivolumab for microsatellite stable (MSS) colo...
- Inoculating Hope: The Landscape of Cancer Vaccination
- Atezolizumab: Investigating Immunotherapy in Head and Neck Squamous Cell Carcinomas (HNSCC) ̵...
Moreover, ALX highlighted the ongoing efforts to understand the mechanism of action underlying evorpacept’s efficacy. Bulk RNA sequencing and multiplex immunofluorescence analyses are being conducted on tissue biopsies collected before and during treatment. These analyses aim to characterize the effects of the treatment on the tumor immune microenvironment, providing valuable insights into its mode of action and potential biomarkers of response.
Looking ahead, ALX announced plans for a Phase II study to further investigate the efficacy of evorpacept in patients with previously untreated indolent non-Hodgkin lymphoma (iNHL). This underscores the company’s commitment to advancing the development of evorpacept across different settings and patient populations.
In conclusion, the data presented by ALX Oncology at the conference provide encouraging evidence of the efficacy and safety of evorpacept in combination with rituximab and lenalidomide for patients with relapsed or refractory B cell non-Hodgkin lymphoma. These findings support further clinical investigation of evorpacept and underscore its potential as a promising treatment option for patients with this challenging disease.
KOL Insights: Dr. Paolo Strati, the lead investigator of the trial, emphasized the significance of these findings, particularly in the context of patients with indolent B-NHL who often experience disease progression after initial treatments. The observed response rates indicate a meaningful clinical benefit for this patient population.
Sophia Randolph, the Chief Medical Officer at ALX, expressed confidence in evorpacept’s differentiated drug design, which has demonstrated anti-cancer activity while minimizing hematologic toxicities associated with other CD47 blocking agents. This suggests a potentially favorable benefit-risk profile for evorpacept compared to existing treatment options.
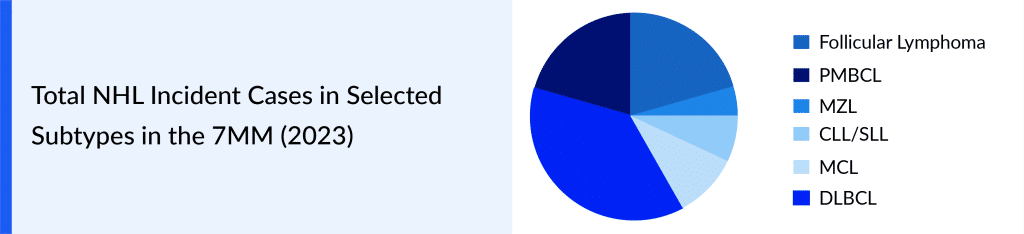
For more information, refer to DelveInsight’s report: Non-Hodgkin’s Lymphoma Market Insight, Epidemiology And Market Forecast – 2032
Downloads
Article in PDF
Recent Articles
- Merck’s ADC SKB264 Shows Promising Results in Advanced Gastric Cancer: Phase 2 Data Reveals Poten...
- Initial results from an open-label phase 1b/2 study of RP1 oncolytic immunotherapy in solid organ...
- AACR 2022: Novartis shifting focus towards directly targeting KRASG12c after disappointment from ...
- Clinical activity of P-BCMA-ALLO1, a B-cell maturation antigen (BCMA) targeted allogeneic chimeri...
- Regeneron Presents Positive Pivotal Data on Linvoseltamab for Relapsed/Refractory Multiple Myelom...