Alzheimer’s Disease: Navigating the Rising Incidence, Evolving Treatments, and Market Dynamics
Feb 25, 2025
Table of Contents
Alzheimer’s disease is more than just memory loss—it’s a progressive neurodegenerative disorder that slowly erodes cognition, behavior, and independence. What may begin as occasional forgetfulness or difficulty recalling names can soon escalate into disorientation, personality changes, and an inability to carry out daily tasks. As the disease advances, patients lose their connection to the world around them, making early detection and intervention crucial.
Recognizing Alzheimer’s symptoms early can help in diagnosis and care planning. Physicians use cognitive tests, brain imaging (PET scans, MRIs), and biomarker analysis of cerebrospinal fluid or blood to confirm an Alzheimer’s diagnosis. These diagnostic advancements have paved the way for more precise and timely interventions, allowing patients to access the latest treatment options sooner.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- The Business Cocktail
- Takeda knocks Velcade; FDA nod awaited; Novartis gets approval; Astellas UK dodges; Haegarda set ...
- GSK consolidates DT and TT vaccine, AZ gears up for FDA filing, Novartis comes up with flex prici...
- How Gene therapy is changing the Beta-thalassemia Treatment outlook?
- Watch Out For Top Pipeline Therapies Making An Impact In The Bipolar Depression Market
The Alzheimer’s Disease Diagnostic Market is primarily driven by advancements in diagnostic technologies such as brain imaging, genetic testing, and blood-based tests. Among these, the computed tomography segment is expected to hold a significant market share due to its ability to detect brain mass loss and differentiate Alzheimer’s from other types of dementia. The increasing launch of CT technologies, such as Philips’ Spectral CT 7500, is further boosting market demand. A rising geriatric population, increasing traumatic brain injuries, and higher Alzheimer’s prevalence are driving North America’s dominance in the AD diagnostic market. These factors are fueling market growth, making the region a promising hub for Alzheimer’s diagnostics.
For years, doctors treated Alzheimer’s disease with cholinesterase inhibitors and NMDA receptor antagonists, which provided temporary relief but failed to slow disease progression. However, a revolutionary shift is underway. Recent FDA-approved Alzheimer’s treatment drugs, including monoclonal antibodies like LEQEMBI and KISUNLA, target amyloid plaques—the root cause of neurodegeneration—offering hope of slowing cognitive decline. With emerging therapies exploring tau protein aggregation, neuroinflammation, and gene-based approaches, the fight against Alzheimer’s is entering a new era—one where stopping the disease may no longer be an impossible dream.
Rising Incidence of Alzheimer’s Disease
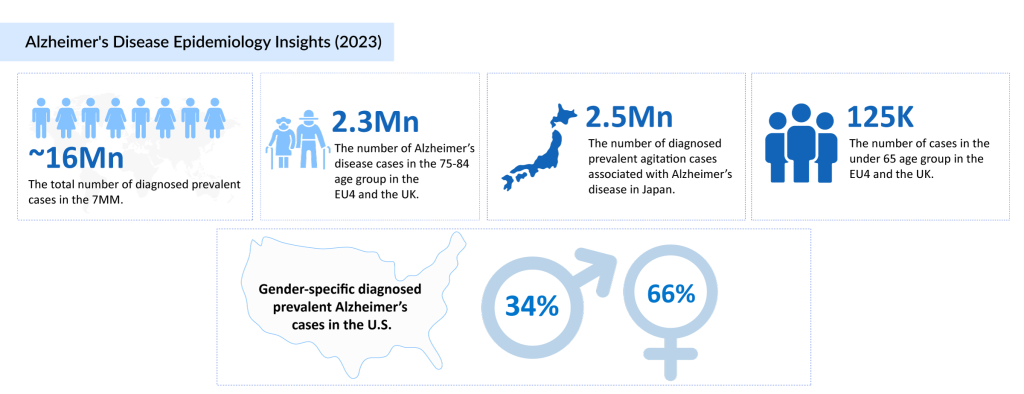
Alzheimer’s disease is a mounting public health challenge, with its incidence rising at an alarming rate worldwide. Currently, an estimated 50 million people are living with Alzheimer’s, and projections indicate that this number will skyrocket to 75 million by 2030 and 131.5 million by 2050, according to Alzheimer’s Disease International. In the seven major markets (7MM), nearly 16 million diagnosed prevalent cases were reported in 2023, with the United States alone accounting for 6.9 million cases. Notably, Alzheimer’s disproportionately affects women, with 4.6 million cases among females compared to 2.3 million in men. Age remains a critical risk factor, with individuals aged 75–84 years making up approximately 44% of the diagnosed cases in the 7MM.
Factors Influencing the Rising Incidence
Several factors contribute to the rising incidence of Alzheimer’s disease, including:
- Aging Population: With increasing life expectancy, the number of individuals at risk is growing.
- Genetics & Family History: People with a family history of Alzheimer’s have a higher likelihood of developing the disease.
- Lifestyle & Environmental Factors: Poor cardiovascular health, sedentary lifestyles, and diets high in processed foods may contribute to cognitive decline.
- Comorbidities: Conditions such as diabetes, hypertension, and obesity are linked to an increased risk of Alzheimer’s.
- Hormonal Changes: Emerging research suggests a potential connection between menopause and Alzheimer’s disease, further increasing the disease burden among women.
Economic Impact of Alzheimer’s Disease
Alzheimer’s disease not only affects individuals and families but also places a significant burden on healthcare systems and economies. The cost of Alzheimer’s care includes:
Direct Costs:
- Long-term skilled nursing care, home healthcare, and hospice services
- Medication therapy management for symptom relief and disease-modifying treatments
Indirect Costs:
- Loss of productivity for patients and caregivers
- Reduced quality of life due to behavioral and cognitive decline
- Informal caregiving burdens, often leading to emotional and financial stress for families
According to the National Library of Medicine, Alzheimer’s treatment costs were estimated at $305 billion in 2020, with projections surpassing $1 trillion by 2050—a staggering financial challenge for global healthcare systems.
The Link Between Menopause and Alzheimer’s Disease
An important discovery in Alzheimer’s research is the link between menopause and cognitive decline. Menopause, which typically occurs between ages 45 and 55, leads to a drastic reduction in estrogen and progesterone levels—hormones that play a crucial role in brain function. Scientists believe this hormonal shift may increase women’s vulnerability to Alzheimer’s disease, making it a critical area of study.
Read our latest blog to explore the connection between menopause and Alzheimer’s disease.
Psychosis in Parkinson’s Disease & Alzheimer’s Disease
Psychosis in neurodegenerative disorders manifests as hallucinations, delusions, and altered perceptions, significantly impacting quality of life. In Parkinson’s disease, psychosis can stem from disease progression or medication side effects, whereas in Alzheimer’s disease, it is more commonly associated with cognitive decline. Diagnosis relies on clinical evaluations, often informed by caregiver observations, highlighting the importance of early detection and intervention.
Agitation in Alzheimer’s Disease: A Critical Challenge
Agitation is a common and distressing symptom of Alzheimer’s disease, characterized by restlessness, aggression, pacing, or verbal outbursts. It often worsens as the disease progresses, affecting both patients and caregivers.
- Biological Factors: Neurotransmitter imbalances, brain structural changes, and neuroinflammation contribute to behavioral disturbances.
- Psychological & Environmental Triggers: Fear, confusion, sleep disturbances, pain, and medication side effects can exacerbate agitation.
- Caregiver Burden: Family members and healthcare professionals often struggle to manage these symptoms, necessitating comprehensive care strategies.
Alzheimer’s disease remains a pressing global challenge, demanding continuous innovation in treatment, early diagnosis, and patient care. Understanding its economic burden, hormonal links, and behavioral manifestations is essential to shaping better healthcare strategies and therapeutic advancements.
How is Alzheimer’s Disease Treated?
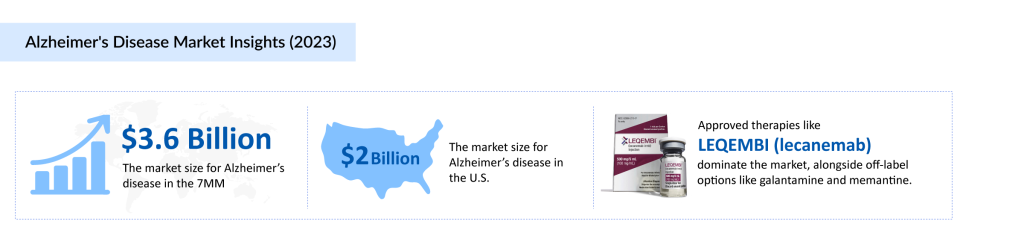
Currently, available therapies for Alzheimer’s disease primarily focus on symptom management by modulating key neurotransmitters such as acetylcholine, serotonin, and noradrenaline while aiming to reduce the activity of glutamate and dopamine. However, these approaches often come with side effects, emphasizing the need for personalized treatment strategies tailored to patient comorbidities and drug interactions. Recent approvals like LEQEMBI (lecanemab) by Biogen and Eisai, KISUNLA (donanemab-azbt) by Eli Lilly, and Brexpiprazole (REXULTI) reflect a shift toward more targeted therapeutic options in the Alzheimer’s disease therapeutics market.
Doctors have prescribed acetylcholinesterase inhibitors (AChEIs) and the NMDA receptor antagonist memantine in the U.S. and worldwide for over a decade. The FDA and EMA have approved three AChEIs—donepezil, rivastigmine, and galantamine—administered in over 60 countries for various stages of Alzheimer’s disease. While these drugs provide modest symptomatic relief, their effects are temporary, requiring regular re-evaluation.
Despite these treatments, challenges remain. The progressive nature of Alzheimer’s disease, its uncertain pathology, and high treatment costs continue to hinder therapeutic advancements. Additionally, the benefit-risk ratio of these medications has led to varied reimbursement policies in countries like France.
Approved Therapies in the Alzheimer’s Disease Treatment Market
The Alzheimer’s disease treatment market includes both FDA-approved therapies and off-label treatments that play a crucial role in managing the disease. Among the most notable therapies is LEQEMBI (lecanemab), developed by Biogen Inc. and Eisai Co., Ltd., which received FDA approval on January 6, 2023. LEQEMBI is a humanized IgG1 monoclonal antibody designed to reduce both soluble and insoluble amyloid beta (Aß) deposits, thereby slowing disease progression. In a significant development, the FDA approved its once-monthly maintenance dosing on January 26, 2025, offering a more convenient option compared to biweekly administration. Additionally, Eisai’s subcutaneous autoinjector version is currently under FDA review, with a PDUFA action date set for August 31, 2025.
Eli Lilly developed KISUNLA (donanemab-azbt), which Japan approved in September 2024. This intravenous infusion (350 mg/20 mL) treats patients with early symptomatic Alzheimer’s disease every four weeks. This approval represents a significant advancement in global treatment accessibility, offering another targeted approach to managing the disease.
Brexpiprazole (REXULTI) has also emerged as an important adjunctive therapy in the treatment landscape. While not directly addressing the neurodegenerative aspects of Alzheimer’s, it is widely used to manage neuropsychiatric symptoms such as agitation, depression, and psychosis, which are commonly observed in patients with moderate to severe Alzheimer’s disease.
These treatments significantly contribute to the Alzheimer’s disease treatment market, which reached USD 3.6 billion in the 7MM in 2023. With several emerging therapies expected to launch between 2024 and 2034, the market is projected to experience substantial growth, driven by innovations in disease-modifying treatments and advancements in precision medicine.
Recent Advancements in the Alzheimer’s Disease Therapies Landscape
The Alzheimer’s disease drugs market is witnessing research and drug development breakthroughs. Several therapies and diagnostic innovations have recently gained regulatory recognition:
Recently, the FDA granted Fast Track designation to troculeucel (Feb 12, 2025), an investigational NK cell therapy by NKGen Biotech, designed to address moderate Alzheimer’s disease. This regulatory recognition aims to accelerate the development of therapies that target the disease’s complex pathology. Additionally, on Jan 26, 2025, Eisai and Biogen received FDA approval for LEQEMBI’s once-monthly maintenance dosing, offering a more convenient treatment option for patients in the Alzheimer’s disease treatment market.
Earlier in the month, on Jan 15, 2025, the FDA granted Breakthrough Device Designation to Spear Bio’s pTau 217 blood test, marking a major step forward in Alzheimer’s disease diagnosis by providing a less invasive alternative to PET scans and lumbar punctures. Furthermore, BioArctic’s LEQEMBI subcutaneous autoinjector is set for an FDA decision by August 31, 2025, potentially expanding patient access to Alzheimer’s disease medicines with a more user-friendly administration method.
These groundbreaking advancements signal a transformative shift toward precision medicine in the Alzheimer’s disease therapeutics market, enhancing both treatment accessibility and effectiveness. With the landscape evolving rapidly, staying informed is crucial.
We’ve curated an exclusive blog covering the latest commercial activities, regulatory updates, and market trends in Alzheimer’s treatment—don’t miss out on these critical insights! Read now and stay ahead of the curve.
Promising Therapies in the Alzheimer’s Disease Pipeline
The Alzheimer’s disease treatment market is set for transformation, with several late-stage investigational drugs showing promising results in the Alzheimer’s disease pipeline:
- Masitinib (AB1010) – AB Science
- An orally administered tyrosine kinase inhibitor targeting neuro-immune cells (mast cells & microglia) involved in Alzheimer’s pathology.
- Phase IIb/III trials have demonstrated its ability to slow cognitive decline, and a confirmatory Phase III trial is underway.
- Valiltramiprosate (ALZ-801) – Alzheon, Inc.
- A small-molecule prodrug designed to block neurotoxic amyloid oligomer formation.
- Currently in Phase III trials, showing improved gastrointestinal tolerance and stable plasma levels compared to its predecessor, tramiprosate.
- Tricaprilin (CER-0001) – Cerecin
- A ketogenic agent designed to provide an alternative energy source to neurons by inducing mild ketosis.
- Aims to counteract declining glucose metabolism in Alzheimer’s patients.
- Remternetug (LY3372993) – Eli Lilly
- Researchers expect a late-stage Phase III monoclonal antibody therapy targeting pyroglutamated amyloid-beta to conclude trials by October 2025.
- If successful, it could become one of the most effective Alzheimer’s disease drugs available, further reinforcing its potential in the Alzheimer’s disease therapeutics market.
As multiple late-stage therapies near approval, the Alzheimer’s disease drugs market advances significantly. Companies like AB Science, Alzheon, AriBio, AgeneBio, Anavex Life Sciences Corp., Cassava Sciences, Novo Nordisk, Eisai, and others are leading research efforts with promising Alzheimer’s disease therapies.

Could Remternetug or other late-stage therapies be the breakthrough the world has been waiting for? Read more about the latest developments here!
Could a Diabetes Drug Help Prevent Alzheimer’s?
Originally developed for type 2 diabetes and weight loss, semaglutide is now gaining attention for its potential to reduce Alzheimer’s disease risk. Research suggests it may protect brain health by improving insulin sensitivity, reducing neuroinflammation, and enhancing brain metabolism.
A recent study published in Alzheimer’s & Dementia found that semaglutide users had a 78% lower risk of developing Alzheimer’s compared to those on other diabetes medications. With Novo Nordisk’s EVOKE and EVOKE+ trials underway, scientists are now investigating whether semaglutide could slow or prevent cognitive decline in non-diabetic individuals.
Could semaglutide redefine Alzheimer’s prevention? Read more about the emerging connection between diabetes and neurodegeneration today!
Future Outlook for Alzheimer’s Disease Treatment
Alzheimer’s disease remains one of the most complex and pressing healthcare challenges of our time. With its rising global incidence, significant economic burden, and devastating impact on patients and caregivers, the demand for effective treatments and preventive strategies has never been greater. For decades, the treatment landscape was limited to symptom management, but the emergence of disease-modifying therapies—such as monoclonal antibodies targeting amyloid plaques—marks a turning point in the fight against neurodegeneration.
Yet, the pursuit of a cure continues. New research into tau-targeting drugs, neuroinflammation, and gene-based therapies is expanding our understanding of Alzheimer’s pathology, while innovative diagnostic tools, such as blood-based biomarkers, are paving the way for earlier and more accurate detection. Meanwhile, unexpected breakthroughs—such as the potential neuroprotective effects of semaglutide, originally developed for diabetes—suggest that Alzheimer’s treatment may also come from unlikely sources, redefining how we approach prevention and care.
As pharmaceutical companies push forward with late-stage clinical trials and regulatory bodies accelerate drug approvals, hope is on the horizon. The Alzheimer’s disease therapeutic landscape is at a pivotal moment, where scientific advancements and therapeutic innovations may soon change the trajectory of this condition. The future of Alzheimer’s care is no longer just about managing decline—it’s about slowing, stopping, and ultimately preventing it.

Downloads
Article in PDF
Recent Articles
- The Business Cocktail
- Novartis buys Medicines; Blackstone Life Sciences and Ferring’s collaboration; Alexion̵...
- FDA’s Ok to Roche’s Oral SMA Therapy; Roche’s Etrolizumab Mixed Results in Ulce...
- Promising therapies that might shift Wet Age-related Macular Degeneration Market in the next decade
- Emergence of Stem cells and its Market Impact