Millions of Patients and Billions in Untapped Androgenetic Alopecia Treatment Market
Feb 03, 2023
Table of Contents
Androgenetic alopecia is a genetic disorder caused by an overreaction to androgens. This condition affects up to 50% of males and females and is characterized by progressive loss of terminal hair on the scalp at any time after puberty. As per Asadi (2020), androgenetic alopecia is the common cause of hair loss in men and women.
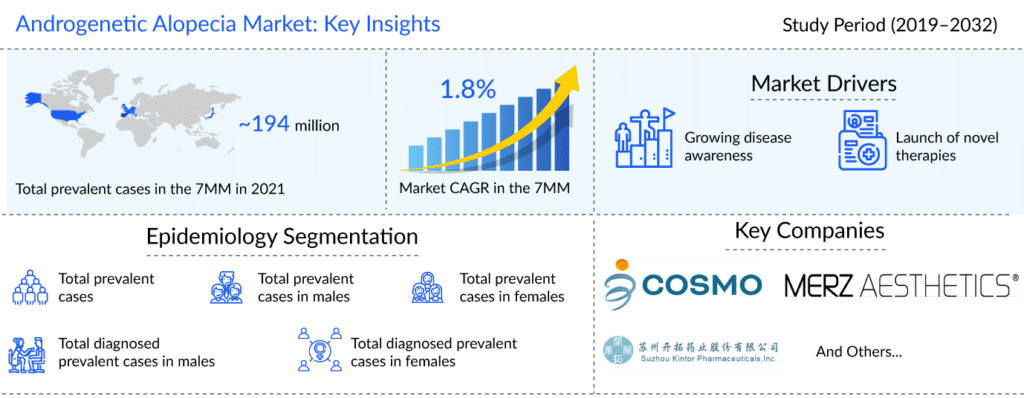
As per the DelveInsight assessment, the total number of prevalent cases of androgenetic alopecia was more than 194 million in the 7MM in 2021. The highest number of cases was seen in EU4 and the UK. Assessments showed that it affected about 50 million men and 30 million women in the United States; it begins in early adolescence and progresses with age. Additionally, more than 50% of men over 50 experience hair loss, whereas, in women, hair loss is more likely to occur after menopause.
Downloads
Article in PDF
Recent Articles
Social Stigma Related to Androgenetic Alopecia
In today’s world, hair plays a big role in how one perceives oneself; therefore, hair loss causes various problems, including poor self-image, diminished confidence, failed relationships in marriage and love, and potential health effects. Studies show that androgenetic alopecia can worsen QoL by contributing to psychological issues such as trauma, anxiety, and depression. Previous studies showed that people with androgenetic alopecia had a much greater prevalence of personality disorders than the general population.
Females face a ton of strain to adjust to pictures utilized by the media and marketing organizations. Be that as it may, the equivalent has likewise become valid for men throughout recent years. Images of actual flawlessness seldom show a man who has lost or is losing his hair.
Looking at oneself too brutally against these unrealistic images causes one to lose certainty and confidence. Having the ‘sculpted physique’ or look comes down on men to conform. Although there are endless male celebrities with bald heads, individuals consider hair loss an issue instead of acknowledging it as a natural process. Additionally, it has been noted that men exhibit more psychopathologic symptoms or a higher prevalence of personality disorders than women.
Minoxidil Ruling the Androgenetic Alopecia Treatment Market
The current androgenetic alopecia treatment regimen mostly consists of oral finasteride, topical external minoxidil usage, low-intensity laser therapy, and hair transplantation. The 5-reductase inhibitor finasteride has a considerable impact on the androgenetic alopecia treatment, but it can also cause unfavorable side effects in a small percentage of patients. Sometimes, adverse androgenetic alopecia symptoms like post-finasteride syndrome continue even after medication is stopped.
Finasteride is approved in males with androgenetic alopecia due to the side effects associated with finasteride; it is generally not recommended for females. Moreover, the risk of a male embryo developing hypospadias should prevent pregnant women from consuming or handling crushed finasteride tablets.
Another drug used for the androgenetic alopecia treatment is dutasteride. It frequently causes erectile dysfunction, decreased semen production, and decreased sex drive as side effects. The drug is possibly more successful than finasteride for treating hair loss, but it also experiences similar rates and adverse effects.
Owing to the side effects of finasteride and other androgenetic alopecia drugs, topical minoxidil is preferred as it does not require a prescription. Minoxidil promotes hair growth by enlarging miniaturized hair follicles, extending anagen, and shortening telogen through an unknown mechanism. In the United States, topical minoxidil is available over the counter in 2% and 5% solutions, as well as 5% foam. When dry, patients should apply 1 mL of 5% solution or half a cap of 5% foam to affected areas of the scalp (not the hair). Treatment efficacy should be evaluated after one year of use, but results may be seen sooner.
Expected Roadblocks in the Androgenetic Alopecia Treatment Market
Although androgens have been identified as the cause of androgenetic alopecia, the etiology and pathogenesis of both male and female pattern hair loss remain unknown. Furthermore, the relationship between androgens and hair loss in women is unclear. Moreover, the scarcity of new epidemiology studies and the dearth of literature on prepubertal androgenetic alopecia, differential diagnosis, and comorbidities associated with pattern hair loss point to the need for ongoing research in these areas.
Furthermore, the widespread use of off-label androgenetic alopecia therapies for the treatment of male and female pattern hair loss, such as dutasteride, prostaglandin analogs, and polyphenols, poses a significant threat to emerging candidates in the androgenetic alopecia treatment space. Moreover, androgenetic alopecia treatment can be especially difficult due to non-uniformity in patient response to conventional therapies, which causes patient non-adherence because they must continue the therapy due to disease relapse after androgenetic alopecia treatment discontinuation.
Optimistic Future of the Androgenetic Alopecia Treatment Market
As androgenetic alopecia is not life-threatening, androgenetic alopecia treatments remain a challenge consisting of a few long-term therapies. These long-term androgenetic alopecia therapies possess several side effects and compliance issues. Moreover, discontinuing the existing androgenetic alopecia therapies can result in the relapse of androgenetic alopecia. Hence, research demanding long-term solutions and permanent androgenetic alopecia treatment are required.
As a result, the androgenetic alopecia treatment market offers a significant opportunity for several players to capitalize on the untapped androgenetic alopecia treatment market. Although the field is quite active and trials to treat patients with androgenetic alopecia are underway, ongoing efforts are required to develop androgenetic alopecia drugs that are both more effective and less toxic. As there are only two approved pharmaceutical androgenetic alopecia treatments across the entire landscape, any significant advancement in this direction is expected to have an impact on the current androgenetic alopecia treatment market scenario during the forecast period (2022–2032).
As per Delveinsight analysis, the androgenetic alopecia market was valued at around USD 5,600 million in 2019 and is expected to reach USD 7,000 million in 2032, growing at a CAGR of 1.81% during the study period.
Companies such as Kintor Pharmaceutical (KX-826) and Cosmo Pharmaceuticals (BREEZULA) are investigating their respective candidates for the management of androgenetic alopecia. Several late-stage assets with distinct MoA are being developed to treat androgenetic alopecia patients and are expected to enter the 7MM androgenetic alopecia treatment market in the next few years.

FAQs
Male and female pattern hair loss (FPHL), also known as androgenetic alopecia (AGA), is a relatively common condition that affects people from all walks of life. Although androgens are the main hormones involved in the pathophysiology of male AGA, FPHL has not always been linked to androgens.
Androgenetic alopecia is caused by a combination of genetics and the effects of male sex hormones known as androgens. Androgenetic alopecia, in particular, is caused by a genetic sensitivity to the androgen DHT, or dihydrotestosterone.
Androgenetic alopecia is typically clinically diagnosed with a history of gradual onset after puberty and, often but not always, a family history of baldness. Unless the androgenetic alopecia diagnosis is unclear, a biopsy is usually not required. Dermoscopy reveals miniaturized hair and brown perihilar casts, which can help distinguish diffuse alopecia areata from male pattern baldness, as diffuse alopecia areata has tapered fractures such as exclamation point hairs.
The two drugs approved by the FDA for androgenetic alopecia are topical minoxidil (ROGAINE) and finasteride (PROPECIA), both of which require at least a 4-6 month trial before noticing improvement and must be used indefinitely to maintain a response. Spironolactone, dutasteride, prostaglandin analogs, laser treatments, cyproterone acetate, platelet-rich plasma, and other off-label therapies are also used to treat androgenetic alopecia. Aside from drug therapies, hair transplantation is a preferred surgical approach for androgenetic alopecia treatment.
As per DelveInsight analysis, in 2021, the total number of prevalent cases of androgenetic alopecia was more than 194 million in the 7MM. The highest number of cases was seen in EU4 and the UK.
Leading companies such as Kintor Pharmaceutical, Cosmo Pharmaceuticals, Merz Aesthetics, and others are currently developing novel therapies for androgenetic alopecia treatment.
Downloads
Article in PDF