Atezolizumab: Investigating Immunotherapy in Head and Neck Squamous Cell Carcinomas (HNSCC) – AACR 2024
Apr 09, 2024
The treatment landscape for locally advanced Head and Neck Squamous Cell Carcinoma (HNSCC) presents a challenge, with conventional approaches such as surgery, radiation, and chemotherapy offering modest success rates. Despite these interventions, less than half of patients achieve disease-free status at 2 years, and 5-year survival rates linger between 40–50%. This underscores the urgent need for novel therapeutic strategies to improve outcomes in this patient population.
Emerging evidence suggests that both chemotherapy and radiotherapy can modulate the tumor immune microenvironment, potentially enhancing PD-L1 expression and thus opening avenues for exploring immune checkpoint inhibitors in patients who have undergone these treatments.
Dr. Deborah Wong, assistant clinical professor of medicine at the David Geffen School of Medicine at UCLA, presented findings from the IMvoke010 clinical trial (abstract CT009) during the Clinical Trial Plenary Session on April 7.
Downloads
Article in PDF
Recent Articles
- Regeneron Presents Positive Pivotal Data on Linvoseltamab for Relapsed/Refractory Multiple Myelom...
- Partner Trial ─ Neoadjuvant Olaparib Disappoints in BRCA Wild-Type TNBC: AACR 2024
- Phase I/II trial of copanlisib in combination with nivolumab for microsatellite stable (MSS) colo...
- Safety and preliminary efficacy of AZD1390 + radiation therapy (RT) for glioblastoma (GBM)
- Inoculating Hope: The Landscape of Cancer Vaccination
The trial, a double-blinded randomized study (NCT03452137), investigated the efficacy and safety of atezolizumab compared to placebo in patients with high-risk locally advanced squamous cell carcinoma of the head and neck, who had undergone definitive local therapy without progression. This study builds upon the promising anti-tumor activity previously observed with atezolizumab in advanced recurrent/metastatic HNSCC, aiming to provide further insights into its potential as a treatment option for locally advanced disease.
However, the results from IMvoke010 revealed that treatment with atezolizumab following multi-modal definitive therapy did not confer a statistically significant improvement in investigator-assessed Event-Free Survival (EFS) compared to placebo. Additionally, there were no discernible differences in EFS or Overall Survival (OS) when assessed by an Independent Review Facility (IRF) between patients receiving atezolizumab versus placebo. Subgroup analyses mirrored the overall findings, indicating consistent outcomes across different patient demographics.
Importantly, the safety profile of atezolizumab remained favorable, with no new safety concerns identified. These findings contribute valuable data to the ongoing discourse regarding the efficacy of checkpoint inhibitors in the locally advanced HNSCC setting. Nonetheless, the role of immunotherapy in this context remains uncertain, warranting further investigation.
In summary, while the IMvoke010 study did not yield the anticipated clinical benefit of atezolizumab in locally advanced HNSCC following multi-modal definitive treatment, its findings underscore the complexities of immunotherapy in this disease setting. Moving forward, continued research efforts are needed to elucidate optimal treatment strategies and identify patient subgroups that may derive maximal benefit from immune checkpoint inhibition.
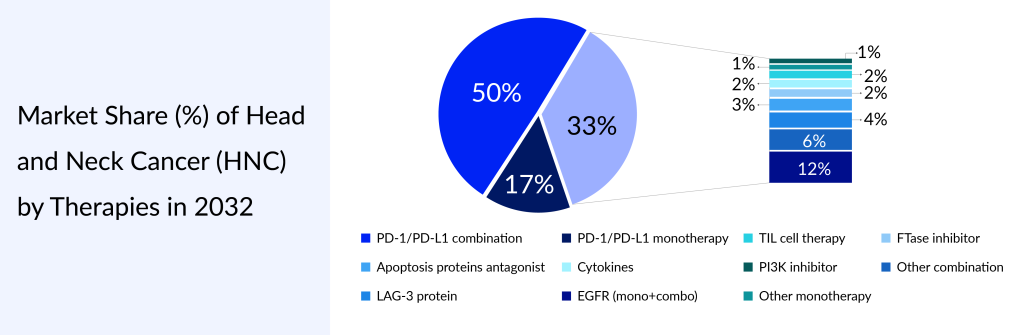
For more information, refer to Delveinsight’s report: Head and Neck cancer (HNC) – Market Insight, Epidemiology And Market Forecast – 2032
Downloads
Article in PDF
Recent Articles
- Personalized vaccine TG4050 induces polyepitopic immune responses against private neoantigens in ...
- CTX130 – CAR T Cell Therapy Offers New Hope for Advanced Clear Cell Renal Cell Carcinoma: A...
- Partner Trial ─ Neoadjuvant Olaparib Disappoints in BRCA Wild-Type TNBC: AACR 2024
- American Association for Cancer Research (AACR) 2023 Preview – Top Data Readouts
- AACR 2022: Novartis shifting focus towards directly targeting KRASG12c after disappointment from ...