Is Bacteriophage a Potential Game-Changer in the Realm of Cystic Fibrosis Treatment?
Jan 12, 2024
Table of Contents
Cystic Fibrosis impacts approximately 1 in every 3,500 births, with around 70,000 affected individuals globally, and 31,000 in the United States alone. Those with the condition experience the development of dense, adhesive mucus in their lungs, making them prone to infections. Over time, this leads to inflammation and a gradual decline in lung function.
DelveInsight estimated that 67,000 individuals were afflicted by cystic fibrosis in 2022. The increase in cystic fibrosis cases underscores the urgent need for innovative therapies as medical advancements have prolonged the lives of cystic fibrosis patients, creating a greater demand for treatments to manage the condition’s long-term effects. Developing novel therapies can address the evolving healthcare requirements of this growing patient population.
Downloads
Article in PDF
Recent Articles
- Dupixent Significantly Reduced COPD Exacerbations; Sosei Heptares to Regain Ownership of GSK43814...
- Cancer-killing virus flees immune destruction and attacks metastatic lung tumors; Urovant’s...
- Ipsen’s Cabometyx Rejected by NICE; Vertex and CRISPR Therapeutics’s Submit BLA to the FDA for ex...
- FDA approval to AZ’s Farxiga for heart failure; GSK divests two travel vaccines to Bavarian Nord...
- Approach for Cystic Fibrosis; An alternative for Statins; Oral medications not safe; Carbon monox...
Domination of Vertex Pharma in the Cystic Fibrosis Treatment Market
The existing treatments for cystic fibrosis aim to alleviate symptoms, prevent complications, and, in recent developments, use protein correctors to address fundamental issues in structure and function. This approach involves using inhaled antibiotics to manage chronic lung infections, mucolytics to reduce the thickness of lung mucus, pancreatic enzyme replacement therapy (PERT) for cystic fibrosis-related pancreatic insufficiency, and CFTR modulators to boost CFTR function, tackling the root cause of the condition.

The primary approach to managing cystic fibrosis involves the use of CFTR modulators and supportive treatments such as mucolytics, antibiotics, bronchodilators, and other therapeutic measures. The FDA-approved drugs of Vertex Pharma – TRIKAFTA, SYMDEKO, ORKAMBI, and KALYDECO dominate the major market share in cystic fibrosis treatment.
The introduction of Vertex Pharma’s Orkambi in January 2012 marked a significant shift in the cystic fibrosis therapeutics market, specifically for patients with the G551D mutation aged six and above. Afterward, Orkambi secured approval in December 2014 for R117H and other gating mutations. Subsequently, in July 2015, Orkambi gained approval for patients aged 12 and above with F508Del. In September 2016, the drug received authorization for patients aged at least 6 years old.
Additionally, in August 2017, Kalydeco, another product by Vertex Pharma, was approved for 23 residual functional mutations. Kalydeco (ivacaftor; VX-770) is a CFTR potentiator indicated for the treatment of cystic fibrosis (CF) in patients aged 6 months and older who have one mutation in the CFTR gene that is responsive to ivacaftor based on clinical and/or in vitro assay data.
Vertex did not halt its efforts, further broadening its cystic fibrosis treatment drug portfolio with the approval of Symdeco in 2018 and Trikafta in 2019. Trikafta is a combination of ivacaftor—a CFTR potentiator—tezacaftor, and elexacaftor indicated for the treatment of cystic fibrosis in patients aged 12 years and older who have at least one F508del mutation in the CFTR gene. Symdeko (Symkevi) is a combination of tezacaftor and ivacaftor, indicated for the treatment of patients with cystic fibrosis ages 6 years and older who are homozygous for the F508del mutation or who have at least one mutation in the CFTR gene that is responsive to tezacaftor/ivacaftor based on in vitro data and/or clinical evidence.
Role of Bacteriophages in the Cystic Fibrosis Treatment Market
Cystic fibrosis results from mutations in the CFTR gene, responsible for encoding a protein that plays a crucial role in regulating the flow of salt and water into and out of cells. In individuals with cystic fibrosis, malfunctioning proteins lead to the accumulation of thick, sticky mucus, which progressively obstructs airways, providing an environment conducive to the proliferation of bacteria and other pathogens. Pseudomonas aeruginosa (PsA) exacerbates the challenge by forming a biofilm in the lungs, impeding the immune system’s ability to independently clear the infection. The majority of those afflicted with this infection ultimately succumb to respiratory failure due to persistent inflammation and infection. The presence of Pseudomonas aeruginosa, along with chronic PsA infection, is linked to a gradual deterioration in lung function. Addressing and managing this organism in the airways of cystic fibrosis patients pose formidable challenges for healthcare providers in cystic fibrosis care.
Managing infections caused by Pseudomonas aeruginosa poses a challenge due to the protective biofilms that prevent effective antibiotic treatment. These biofilms not only act as a barrier to antibiotics but can also evolve, leading to increased resistance. Despite advancements in treatments targeting CFTR, patients with cystic fibrosis still grapple with persistent and untreatable infections, particularly those attributed to Pseudomonas aeruginosa. Consequently, there is a pressing demand for innovative treatment strategies to address this issue. Bacteriophages have been suggested as a substitute for antibiotics, offering the potential for exceptionally targeted efficacy against harmful pathogens.
Emerging Therapies Based on Bacteriophages for Cystic Fibrosis Treatment
Companies across the globe are diligently working toward the development of novel treatment therapies with a considerable amount of success over the years. Market key players, such as BiomX, Inc. (BX004), Aridis Pharmaceuticals, Inc. (AR-501), Respirion Pharmaceuticals Pty Ltd (RSP-1502), and others are developing drugs for the treatment of cystic fibrosis.
BiomX is in the process of developing BX004, using its exclusive BOLT platform, to address chronic pulmonary infections in cystic fibrosis patients caused by P. aeruginosa—a significant contributor to morbidity and mortality in cystic fibrosis cases. In September 2021, the FDA granted clearance for the initiation of a Phase Ib/IIa study on BX004 for cystic fibrosis patients dealing with chronic pulmonary infections related to P. aeruginosa. The Phase Ib/IIa trial consisted of two segments. The first part assessed the safety, pharmacokinetics, and microbiologic/clinical activity of BX004 in a single ascending dose and multiple dose design involving nine cystic fibrosis patients. The second part involved the evaluation of the safety and efficacy of BX004 in 34 cystic fibrosis patients randomly assigned to either treatment or placebo in a 2:1 ratio. Positive top-line results from the second part were disclosed on November 29, 2023. In August 2023, the FDA granted Fast Track designation to BX004 for the treatment of chronic pulmonary infections caused by P. aeruginosa bacterial strains in cystic fibrosis patients.
Additionally, a late-breaking abstract by BiomX Inc., presented data from Part 1 of its ongoing Phase Ib/IIa (NCT05010577) study to evaluate BX004, a new phage product candidate, for the treatment of chronic Pseudomonas aeruginosa (PsA) lung infection in patients with cystic fibrosis at the 2023 International Congress of the European Respiratory Society (ERS).
Recently, on Jan 04, the FDA granted Orphan Drug Designation (ODD) to BiomX’s BX004. This significant milestone offers optimism for individuals dealing with cystic fibrosis, particularly those facing persistent pulmonary infections triggered by Pseudomonas aeruginosa. BiomX’s pioneering contributions to cystic fibrosis treatment are unquestionably transforming the healthcare sector. With the ODD and Fast Track designation for BX004, the company is on the brink of ushering in a new era of hope and enhanced quality of life for patients grappling with this incapacitating condition.
RSP-1502 developed by Respirion Pharmaceuticals Pty Ltd is a blend of an FDA-approved antibiotic (tobramycin) and a unique biofilm disruption agent, specifically designed for inhalation through a highly efficient vibrating mesh nebulizer. This nebulizer not only ensures a quicker treatment process, lasting only 5-10 minutes but also enhances patient inhalation. The efficacy of RSP-1502 was validated through a human pilot study, conducted in collaboration with the Telethon Kids Institute and the Health Department of Western Australia. The results demonstrated enhanced bacterial clearance and improved lung function in individuals treated with RSP-1502 compared to those who received a placebo along with tobramycin.
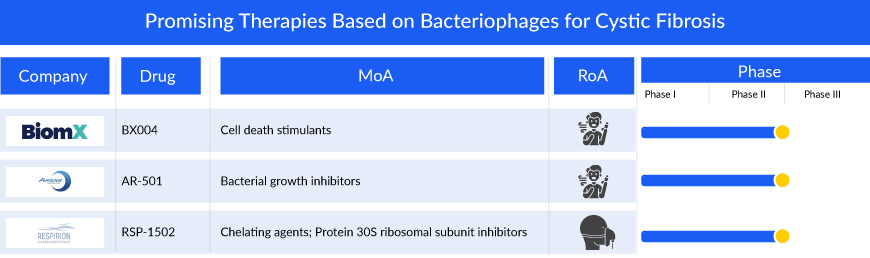
AR-501 represents a new type of small molecule anti-infective, adding diversity to Aridis Pharmaceuticals, Inc.’s product portfolio primarily centered on monoclonal antibody immunotherapy. This inhalable version of gallium citrate serves as an iron analog, aiming to deprive bacteria of iron. The mechanism involves gallium inhibiting various iron-dependent synthetic and metabolic pathways essential for the pathogenicity of bacteria.
AR-501 is currently in development as an inhalation therapy aimed at treating life-threatening bacterial lung infections in individuals with cystic fibrosis. A Phase I/IIa clinical trial has recently concluded, involving healthy adult volunteers and adult patients with cystic fibrosis. The inhaled administration of AR-501 demonstrated good tolerance among healthy adult volunteers (totaling 48 participants), encompassing five consecutive weekly doses across all tested dosage levels (6.4mg, 20mg, and 40mg). Notably, there were no instances of serious adverse events (SAE), and all observed adverse events (AE) were of Grade 1 or Grade 2 severity.
Results from the Phase IIa cystic fibrosis trial involving 41 cystic fibrosis patients indicated favorable outcomes with inhaled AR-501. The treatment was well-received across all repeated weekly doses and various tested dose levels, with no occurrences of serious adverse events linked to the drug. Adverse events were primarily of Grade 1 or Grade 2 severity. Notably, AR-501 demonstrated significant uptake in the respiratory tract, as evidenced by sputum drug concentrations, surpassing levels more than 50 times higher than necessary to inhibit the target bacteria P. aeruginosa. Moreover, the quantity of AR-501 delivered to the lungs exceeded that achieved through intravenous administration in a Phase II study, where improvements in lung function were observed in cystic fibrosis patients, with concentrations over 10 times higher.
Future Cystic Fibrosis Treatment Space is Promising
The future of cystic fibrosis treatment holds promising advancements, with researchers exploring innovative therapies to improve the quality of life for individuals with this genetic disorder. Among the emerging treatment modalities, bacteriophage-based therapies are garnering attention for their potential in addressing the persistent bacterial infections that often afflict cystic fibrosis patients.
DelveInsight’s analysis revealed that the estimated current market size of cystic fibrosis reached USD 8.3 billion in 2022 and it is expected to grow at a significant CAGR by 2032 owing to the expected launch of potential therapies, along with increasing awareness and development of novel therapies.
Moreover, bacteriophages are viruses that specifically target and infect bacteria, offering a highly targeted approach to combatting harmful pathogens in the lungs of cystic fibrosis patients. Unlike traditional antibiotics, bacteriophages can adapt to evolving bacterial strains, potentially providing a more sustainable and effective solution. As research progresses, the integration of bacteriophage-based therapies into the cystic fibrosis treatment landscape could revolutionize how healthcare professionals approach and manage bacterial infections in individuals with cystic fibrosis, offering new hope for improved outcomes and enhanced quality of life.

Downloads
Article in PDF
Recent Articles
- Vertex/CRISPR’s Gene-editing Therapy exa-cel: Inch Ahead of Rival
- Dupixent Significantly Reduced COPD Exacerbations; Sosei Heptares to Regain Ownership of GSK43814...
- Symdeko’s approval; Erytech target solid tumors; Oncologie snapped bavituximab; Merck delivers
- Amgen to Purchase Horizon Therapeutics; IND Clearance to Vertex’s VX-522; FDA Fast-Track Designat...
- Bridging the gap: Nontuberculous mycobacterium Infections Drug Pipeline



