Biologics Spark Market Interest In The Nasal Polyposis Treatment Space
Feb 10, 2023
A highly prevalent condition, Nasal polyps are benign growths in the mucosa of the nose that blocks the nasal passageway, reducing the quality of life (QoL), causing hyposmia or anosmia and sleep disturbances in those affected. Often associated with chronic nasal inflammation and conditions like asthma, these are a common subgroup of chronic rhinosinusitis (CRS). Chronic rhinosinusitis with nasal polyps (CRSwNP) is a potentially debilitating condition in adults, and approximately 25% of diagnosed CRS individuals have an associated nasal polyp condition.
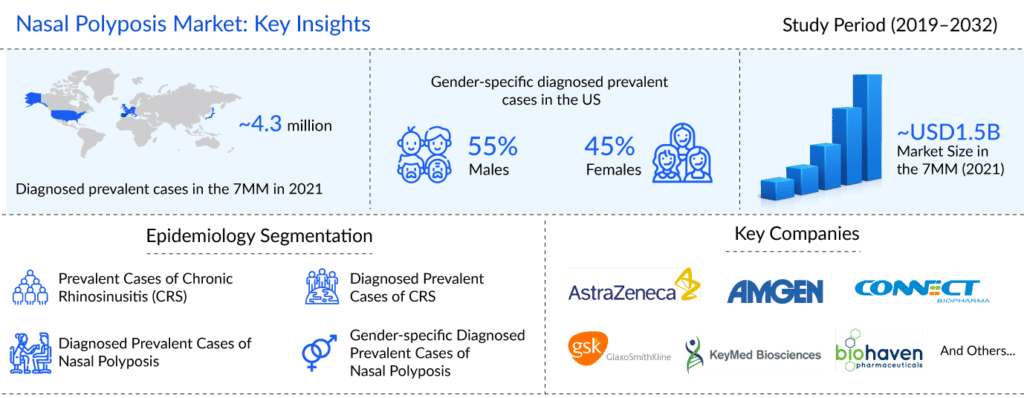
According to DelveInsight’s estimates, out of 8.7 million diagnosed prevalent cases of CRS, there were approximately 2.1 million cases with nasal polyps in the US in 2021. Among these cases, 55% of cases were male. In EU-4, Germany had the highest diagnosed cases of nasal polyposis, followed by France in 2021. While Spain had the least number of cases.
The Current Nasal Polyposis Treatment Landscape
The primary objective of the current nasal polyposis treatment is to slow the growth of the polyps, delay surgery, or prevent relapse after surgery. Reoccurrences are common and high. Due to unclear etiology in most cases, nasal corticosteroids are usually the go-to treatment for nasal inflammation. If the patient does not show improvement, injectable corticosteroids may be given.
Downloads
Article in PDF
Over the years, the US FDA has approved various corticosteroids for nasal polyposis treatment, which include XHANCE (fluticasone propionate), SINUVA (mometasone furoate) Sinus Implant, PROPEL (mometasone furoate), but these are not curative, leaving the patients vulnerable. In addition, depending on the patient’s needs, antihistamines, antibiotics, and NSAIDs may be administered. In many cases, patients not responsive to steroids or have large polyps may require surgical intervention.
The recent approval of biologics as add-on therapy has provided optimism for patients with cystic fibrosis. There are currently three approved biologics for nasal polyposis treatment, which include DUPIXENT (dupilumab), NUCALA (mepolizumab), and XOLAIR (omalizumab). Others like FASENRA, CM310, TEZSPIRE (tezepelumab), and Depemokimab (GSK3511294) are also being developed, offering a robust biologics nasal polyposis pipeline.
Experts say biologics are considered before primary surgery, particularly in patients whose conditions like asthma are present simultaneously with CRSwNP, in those for whom surgery is less available, in patients who refuse surgery, and in patients with a high complication ratio. The use of nasal polyposis biologics straightaway after surgery might be considered for patients whose nasal polyps recur a year after the surgery.
Regeneron’s broad labeled mAb, DUPIXENT (dupilumab), was the first biologic approved by the US FDA in 2019. Roche’s XOLAIR and GSK’s NUCALA made their entrance soon after in 2020 and 2021, respectively. However, DUPIXENT leapfrogged into a clear nasal polyposis treatment market leader, outperforming its competitors in head-to-head comparisons in efficacy nasal polyposis clinical trials. It demonstrated exceptional results with a 35% reduction in nasal polyp scores compared to XOLAIR’s 18% and NUCALA’s 15% reductions, respectively. In the Sinonasal Outcome Test (SNOT-22), DUPIXENT had a 43% reduction compared with XOLAIR’s 21% and NUCALA’s 27% reduction. DUPIXENT also had the best improvements in the degree of nasal congestion severity. These results have given DUPIXENT all the edge it needs to dominate the nasal polyposis treatment market.
Scope of Upcoming Therapies for Nasal Polyposis Treatment
While the current players are celebrating their wins in this nasal polyposis treatment space, newer players hope to trail their own success. There are a few emerging nasal polyposis therapies in the clinical pipeline awaiting approval. Fasenra is one such therapy whose application has been accepted by the FDA in the United States for nasal polyposis treatment. Although it is too early to predict that emerging nasal polyposis therapies, such as Fasenra and tezepelumab, will be successful and generate large revenues, their nasal polyposis treatment market acceptance appears to be promising, as regulatory authorities have approved biologics. These nasal polyposis drugs are likely to succeed in their respective clinical trials and have a positive impact on the nasal polyposis treatment landscape in the 7MM during the study period (2019–2032).
AstraZeneca/Amgen’s TEZSPIRE (tezepelumab), a first-in-class monoclonal antibody that targets an upstream master cytokine, thymic stromal lymphopoietin (TSLP), will be the only biologic with no phenotypic (eosinophilic or allergic) or biomarker restriction in its authorized label. GSK will fortify its portfolio with Depemokimab, another long-acting IL-5 antagonist like NUCALA, while Keymed Biosciences’ CM310, a IL-4Rα antagonist like DUPIXENT, will cash in on its success.
Optimistic Future of the Nasal Polyposis Treatment Market
The overall nasal polyposis treatment market is expected to boost due to the rising prevalence of nasal polyposis over the globe and, thus, the surge in nasal polyposis treatment options. Along with these, the expected launch of emerging therapies will boost the nasal polyposis treatment market.
As per DelveInsight analysis, the nasal polyposis market size in seven major markets was approximately USD 1,531 million in 2021, which is further expected to increase by 2032 at a Compound Annual Growth Rate (CAGR) of 9.05% for the study period (2019–2032).
Furthermore, many pharma companies are working to assess challenges and seek opportunities that could influence nasal polyposis R&D, resulting in a plethora of potential therapies centered on novel approaches to treat nasal polyps. The approval of these emerging therapies are expected to have a significant impact on the global nasal polyps treatment market.
The current nasal polyposis pipeline includes biologics, benralizumab, tezepelumab, and others in the mid and late stages of clinical development that are awaiting approval. Previous nasal polyposis treatment strategies had a significant unmet need; many patients frequently relapsed from these interventions. As a result, the development of new entities was required. The introduction of biologics has provided a reason for optimism. As the nasal polyposis treatment market becomes more competitive, it will be interesting to see how these emerging therapies fair against each other in the nasal polyposis treatment market.
On the other hand, misdiagnosis of nasal polyposis can act as a hurdle to the nasal polyposis treatment market growth. Moreover, the tools to define disease severity are also not well established. In addition, the lack of cost-effective nasal polyposis treatment, lack of robust clinical nasal polyposis pipeline, and limited awareness of nasal polyps can limit the nasal polyposis treatment market growth in the coming years.
FAQs
Nasal polyposis is a small, benign (noncancerous) drop-like growth that appears in the mucosa (lining tissues) of the nose and can obstruct the nasal passageway. Ethmoidal polyps and Antrochoanal polyps are two nasal polyps types. The most common type is ethmoidal polyps.
Some of the nasal polyps symptoms include runny nose, stuffiness, or post-nasal drip. There may be no nasal polyps symptoms in some cases.
Researchers are unsure what causes nasal polyps. Nasal polyps are thought to be caused by a variety of factors. It is thought that the inflammation is caused by fluid buildup in the mucus membrane. This buildup causes fluid-filled growths in the nasal or sinus passage, which grow into polyps over time. Nasal polyps are commonly found in the sinus area near the eyes, nose, and cheekbones.
An ENT specialist or other physician will first look into the nasal passages with a special lighted instrument known as a nasoscope to make the nasal polyps diagnosis. Nasal endoscopy and radiological imaging are the most commonly used techniques for confirming the location, size, and severity of polyps. Additional allergy and blood tests can be performed to determine other disease-related components.
Currently, corticosteroids (intranasal and systemic) and biologics (Dupixent, Xolair, and Nucala) can be used for Nasal Polyps treatment. Corticosteroid implants, such as Propel and Sinuva, are also available to improve surgical outcomes and to treat recurrent Nasal Polyps. In addition, depending on the patient’s needs, antihistamines, antibiotics, and NSAIDs may be administered. Surgery to remove the polyps can be performed if therapeutic agents do not improve the patient’s condition.

Downloads
Article in PDF



