Bristol Mayer Squibb — The Undisputed Leader in the Molecular Glue Arena
Jan 13, 2025
Table of Contents
Bristol Myers Squibb stands tall as the undisputed leader in the molecular glue arena, shaping the future of targeted therapies. With its groundbreaking advancements, the company has redefined the potential of molecular glues in oncology, offering innovative solutions that precisely degrade harmful proteins. Bristol Myers Squibb’s relentless commitment to scientific excellence and transformative research positions it at the forefront of this rapidly evolving field, paving the way for novel, life-changing treatments that address some of the most complex diseases.
Bristol Myers Squibb — Dominating the Molecular Glue Sector
Bristol Myers Squibb headquartered in New York City is a global biopharmaceutical company that focuses on discovering, developing, and delivering innovative medicines to patients with serious diseases. Currently, BMS is the only company with approved products and enjoying this monopoly in the molecular glue market. The approved molecular glues developed by BMS are REVLIMID, POMALYST, and Thalomid.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- CELMoDs – A Worthy Successor to REVLIMID?
- Syndax Pharmaceuticals Receives FDA Approval for REVUFORJ; FDA Approves BLENREP for Adults with R...
- Merck Wins FDA Approval for KEYTRUDA QLEX for Subcutaneous Use in Adults With Solid Tumors; Incyt...
- TALVEY’s Versatility Shines: Key Insights from MonumenTAL-2 Study Illuminate Effective Stra...
- Cilta-Cel’s Prolonged Impact: Deep Responses and Safety Insights from Earlier Lines of Ther...
REVLIMID is a prescribed medication for treating adults with multiple myeloma in combination with dexamethasone or as a maintenance treatment following autologous hematopoietic stem cell transplantation. It should not be used in individuals with chronic lymphocytic leukemia unless they are part of a controlled clinical trial. The safety and effectiveness of REVLIMID in children are not known.
POMALYST is a prescribed medication used to treat adults with multiple myeloma, in combination with dexamethasone, for patients who have already been treated with at least two other medications, including a proteasome inhibitor and lenalidomide, and whose condition worsens during or within 60 days of completing the last treatment. The safety and effectiveness of POMALYST in children have not been established.
Thalomid (thalidomide) is a medication developed by Bristol Myers Squibb primarily used in the treatment of multiple myeloma, a type of blood cancer, and erythema nodosum leprosum, a complication of leprosy. Initially introduced in the late 1950s, it was withdrawn from the market due to its severe teratogenic effects, which caused birth defects.
However, after rigorous studies, Thalomid was re-approved in the 1990s for its therapeutic benefits in specific conditions. It works by modulating the immune system and inhibiting the growth of blood vessels that supply tumors, playing a key role in the treatment of multiple myeloma. Thalomid’s use is strictly controlled due to its risks, particularly during pregnancy, but it remains an important part of the treatment regimen for multiple myeloma, often used in combination with other chemotherapy agents.
Explore more about molecular glues at “The Rise of Molecular Glues: Transforming Protein-Protein Interactions into Therapeutic Opportunities”
Upcoming Molecular Glues in the BMS Pipeline
Despite being the only company with approved products in the molecular glue space, BMS is further strengthening its dominance by evaluating more molecular glue assets. Currently, the BMS’ molecular glues pipeline consists of 3 candidates — Mezigdomide, Golcadomide, and Iberdomide. All these drugs are in the late stage of development.
Mezigdomide
Cereblon E3 ligase modulators (CELMoD) are a type of oral immunomodulatory therapy developed to boost the immune system and directly target cancer cells by promoting the degradation of tumor-supporting proteins. Bristol Myers Squibb is exploring a new CELMoD drug, mezigdomide, for multiple myeloma. This agent was specifically designed to enhance the effectiveness of IMiD agents while maintaining manageable side effects, easy administration, and the potential to improve patient outcomes. Mezigdomide leverages cereblon to quickly trigger the degradation of target proteins Ikaros and Aiolos, thereby inhibiting tumor cell growth, encouraging tumor cell death, and activating immune-stimulatory effects.
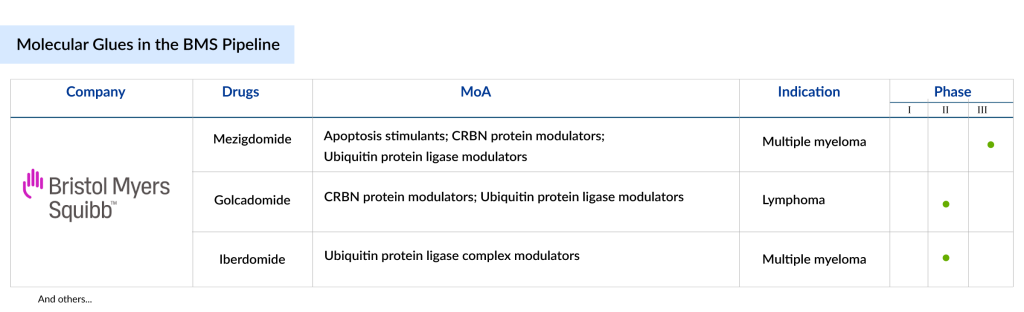
Golcadomide
Golcadomide (CC-99282) is an oral molecular glue that facilitates the degradation of IKZF1/3 transcription factors (Ikaros/Aiolos) by interacting with the cereblon E3 ubiquitin ligase complex. It is a new thalidomide analog, classified as a CELMoD (cereblon E3 ligase modulator). Golcadomide exhibits immunomodulatory effects, with improved antiproliferative and proapoptotic properties. Currently, it is in Phase III development for the treatment of B-cell lymphoma.
Iberdomide
Iberdomide is an experimental oral immunomodulatory medication being developed for the treatment of multiple myeloma, a blood cancer marked by the growth of abnormal plasma cells in the bone marrow. A derivative of thalidomide, Iberdomide works by modulating the immune system and targeting the cereblon E3 ligase complex, which leads to the breakdown of specific proteins that support cancer cell survival and growth. This mechanism makes Iberdomide a promising treatment option in a field that includes other immunomodulatory drugs such as lenalidomide and pomalidomide. In December 2018, the US FDA designated iberdomide as an orphan drug for the treatment of multiple myeloma. The drug is undergoing Phase III clinical trials for treating multiple myeloma.
Once approved, all these therapies will further give BMS an upper hand in the molecular glue landscape.
Other Companies Developing Molecular Glues
Apart from BMS, several other pharma companies are also trying their luck to enter the molecular glue arena and give stiff competition to the current market leader. Currently, 50+ companies are working on their lead assets, although all these molecular glues are still in the early stages yet they have the potential to change the molecular glue competitive landscape.

The molecular glue companies evaluating their products include Eisai Co Ltd (E 7820), Monte Rosa Therapeutics, Inc. (MRT-2359), Revolution Medicines, Inc. (RMC 6291 and RMC-6236), InnoCare Pharma (ICP-490), C4 Therapeutics (CFT 7455), Nurix Therapeutics, Inc. (NX-5948 and NX-2127), Gluetacs Therapeutics (GT919), Plexium (PLX-4545), Novartis Pharmaceuticals (DKY709), Nested Therapeutics (NST-628), Salarius Pharmaceuticals, Inc. (SP-3164), and others.
What Lies Ahead?
Molecular glues are set to revolutionize drug discovery, particularly in addressing previously “undruggable” targets, and Bristol Myers Squibb has positioned itself as a leader in this cutting-edge space. Molecular glues work by facilitating interactions between proteins, often targeting disease-causing proteins for degradation. BMS, with its strong track record in protein degradation therapies, exemplified by its development of cereblon-modulating compounds like lenalidomide and pomalidomide, is leveraging its expertise to expand into molecular glues. By investing in advanced technologies and forging collaborations, BMS is not only building a robust pipeline but also establishing proprietary platforms that could dominate this therapeutic class. These efforts are likely to give BMS a significant competitive edge in offering first-in-class or best-in-class therapies across oncology, immunology, and other areas.
However, BMS is not the only player in the molecular glue race, and stiff competition from other pharmaceutical companies could challenge its lead. Rivals such as Novartis, Amgen, and smaller biotech firms like Arvinas and Kymera Therapeutics are also heavily investing in the development of molecular glues. These companies bring diverse approaches, including proprietary discovery platforms, partnerships with academic institutions, and breakthroughs in understanding protein-protein interactions. Additionally, new entrants and startups, often fueled by venture capital and innovative research, are accelerating the pace of innovation. This competitive landscape means that BMS must continuously innovate, secure intellectual property, and execute its pipeline effectively to maintain its leadership, as the potential of molecular glues attracts increasing attention and investment across the pharmaceutical industry.

Downloads
Article in PDF
Recent Articles
- 5 Most Promising CAR T-cell Therapies for Multiple Myeloma in Development
- Analyzing the Most Promising Drugs That Will Lose Patent in the US & EU in 2022
- Intensity Therapeutics Publishes Compelling Clinical Data for INT230-6 in Advanced Cancers; UCB W...
- Pfizer to acquire Arena Pharma; Takeda’s ‘Wave 2’ multiple myeloma med data; No...
- Pfizer/ OPKO Health’s Somatrogon; FDA EU for SCONE device; Antengene ATG-010 for rrMM and rrDLBCL...



