Breakthrough Therapies Shaping the Future of Acute Kidney Injury Treatment
Jan 16, 2025
Table of Contents
Acute kidney injury is a sudden and severe condition characterized by the rapid loss of kidney function, resulting in the accumulation of waste products in the blood and imbalances in fluids and electrolytes. It is often caused by factors such as severe dehydration, infections, certain medications, or complications from surgery. AKI affects an estimated 13.3 million people worldwide each year, with a higher prevalence in hospitalized patients and those in critical care settings. The condition can occur across all age groups but is more common in older adults and individuals with underlying chronic conditions. While AKI can often be reversed with timely treatment, severe cases may lead to chronic kidney disease or even kidney failure.
As the understanding of Acute kidney injury evolves, research into innovative treatments and preventive measures is accelerating. Advances in medical science are driving the development of therapies aimed at mitigating the damage to kidney cells, improving recovery outcomes, and preventing long-term complications. From biomarker-based diagnostic tools to regenerative therapies and novel drug formulations, researchers and healthcare providers are exploring ways to enhance patient outcomes. Emerging therapies focus on reducing inflammation, improving renal perfusion, and protecting kidney function, offering hope for better management of AKI in the future.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Infectious Disease and Vaccines Pipeline Reports
- Oral Mucositis – A Common Cancer Therapy Adverse Reaction
- Breakthrough Device Designation granted to the AI Software for CTEPH
- Bubble Boy Syndrome (ADA-SCID) – A Rare Immunodeficiency Disorder
- Systemic Juvenile Idiopathic Arthritis -Pipeline Insights, 2015
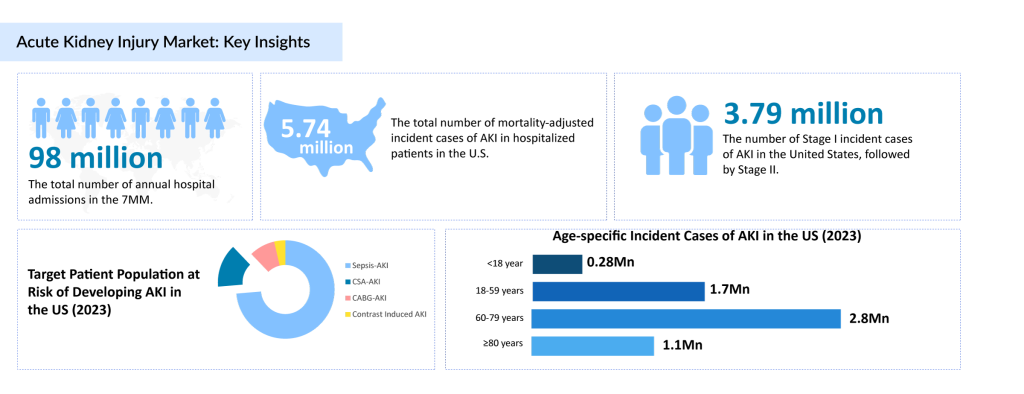
Current Landscape of Acute Kidney Injury Treatments
Acute kidney injury is a significant global health issue, with management heavily reliant on supportive therapy such as renal replacement therapy (RRT) and off-label acute kidney injury medications like ACE inhibitors, ARBs, diuretics, and NSAIDs. Despite their widespread use, these treatments do not address the underlying causes of AKI, leaving healthcare providers with limited tools for effective management of acute kidney injury. This reliance on conventional approaches underscores a critical unmet need for targeted acute kidney injury treatments that can improve patient outcomes and address the condition’s root causes.
The market for acute kidney injury treatments was valued at approximately USD 6.2 billion in 2022 across the 7MM (United States, EU4, UK, and Japan). The United States leads this market due to a high burden of disease, robust healthcare infrastructure, and significant investment in research and development. Among European nations, Germany commands the highest revenue, while Spain has the lowest market share. Japan’s acute kidney injury medication market reached a value of USD 35 million in 2022 and is set to grow significantly between 2024 and 2034. Innovations in AKI therapies and increasing awareness of effective AKI management are driving the market’s projected CAGR of 4.1%.
Emerging Therapies for Acute Kidney Injury Treatment
As there are no approved drugs for the treatment of AKI, companies are working in the pipeline constantly. The treatment landscape is evolving, supported by a strong pipeline of innovative therapies that aim to redefine AKI management. These emerging solutions target specific mechanisms, such as inflammation, oxidative stress, and renal cell damage, to improve outcomes in acute kidney injury patients. Prominent candidates include Renibus Therapeutics’ RBT-1, which focuses on oxidative stress and renal protection, and Ocelot Bio’s OCE-205, addressing hepatorenal syndrome-associated AKI. Additionally, AM-Pharma’s Ilofotase alfa is exploring the potential of alkaline phosphatase in promoting kidney recovery, while Guard Therapeutics’ RMC-035 aims to protect kidneys during high-risk surgical procedures. These advancements represent a paradigm shift in treatment for AKI, moving beyond traditional supportive therapy to directly target the disease mechanisms.
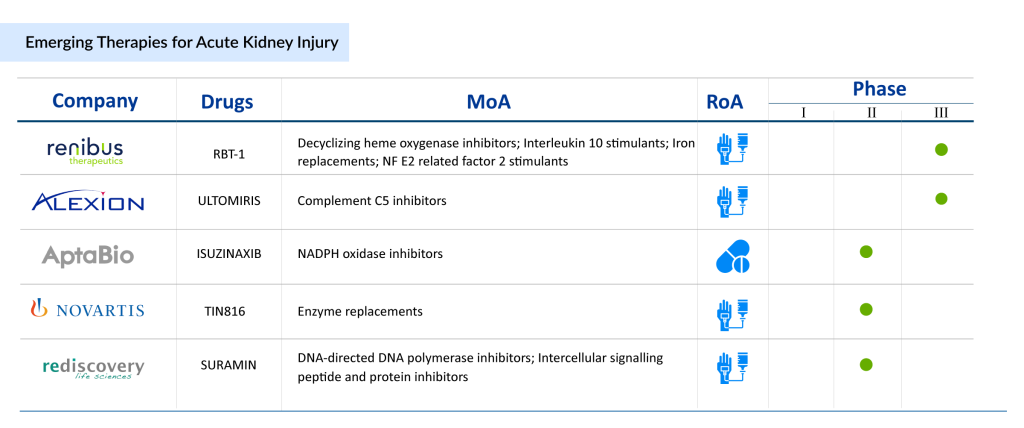
The emergence of therapies targeting complications such as sepsis-induced AKI, cardiac surgery-associated AKI, and delayed graft function further underscores the evolving market dynamics. These developments offer hope for better patient outcomes, reduced mortality rates, and improved quality of life. As advancements continue, the future of the management of acute kidney injury looks increasingly optimistic, with groundbreaking therapies set to transform the standard of care for patients worldwide. The next decade promises significant strides in addressing the unmet needs of the acute kidney injury patient population.
Let’s take a deeper look at each of these promising therapies for AKI. We’ll explore their mechanisms, clinical progress, and potential impact on improving patient outcomes. By examining these therapies thoroughly, we can better understand the future of AKI management.
Renibus Therapeutics’ RBT-1
Phase – III
RBT-1 is an investigational drug developed by Renibus Therapeutics, designed to activate a cytoprotective preconditioning response that upregulates anti-inflammatory, antioxidant, and iron-scavenging pathways. This mechanism aims to reduce postoperative complications, particularly following cardiothoracic surgery, by protecting vital organs, including the kidneys, from damage. By mitigating risks like acute kidney injury, RBT-1 has the potential to improve patient outcomes after high-risk surgeries.
In January 2024, Renibus Therapeutics published the results of a Phase II trial that evaluated RBT-1 as a preconditioning agent in patients undergoing cardiothoracic surgery. The trial results showed that RBT-1 could significantly reduce postoperative complications, suggesting a promising role for the drug in improving recovery and reducing the incidence of kidney-related issues after surgery. These findings supported further exploration in more extensive clinical trials.
Building on this momentum, Renibus announced in October 2023 the dosing of the first patient in the pivotal Phase III PROTECT trial. This trial aims to confirm the safety and efficacy of RBT-1 in a larger patient population, with top-line results expected by mid-2025. In recognition of its potential, the U.S. FDA granted Breakthrough Therapy Designation to RBT-1 in July 2023, accelerating its development for reducing postoperative complications in cardiothoracic surgery. The company plans to file the NDA by early 2026, moving RBT-1 closer to becoming a viable treatment option.
AM Pharma’s Ilofotase alfa
Phase – III
Ilofotase alfa, developed by AM-Pharma, is a recombinant alkaline phosphatase designed to protect the kidneys, especially in patients with sepsis-associated acute kidney injury (SA-AKI). By detoxifying harmful molecular patterns that contribute to kidney damage, Ilofotase alfa showed promising results in Phase II trials, with a 46% reduction in mortality compared to placebo. These findings led to the initiation of the REVIVAL Phase III trial, aimed at confirming its efficacy in reducing 28-day all-cause mortality and improving kidney function in SA-AKI patients.
The REVIVAL trial, currently ongoing, is enrolling 1,400 patients across North America, Europe, and Japan. Although the trial was stopped for futility on the primary endpoint, the study continues with interim analyses to assess other key outcomes. Alongside this pivotal trial, AM-Pharma is conducting a Phase I study in Japan to evaluate the pharmacokinetics, safety, and tolerability of Ilofotase alfa. If successful, this drug could offer a significant advancement in the treatment of acute kidney injury caused by sepsis and other comorbidities.
Ocelot Bio’s OCE-205
Phase – II
Ocelot Bio’s lead asset, OCE-205, is a therapeutic peptide with a unique mechanism of action aimed at treating hepatorenal syndrome (HRS)-associated acute kidney injury (AKI). OCE-205 works by eliminating V2 receptor activity, which helps prevent fluid retention and overload, a common issue in patients with HRS. A Phase II clinical trial is currently evaluating the drug’s effectiveness in improving kidney function for these patients, who often face limited treatment options.
In August 2022, Ocelot Bio received an Orphan Drug Designation (ODD) from the U.S. FDA for OCE-205, recognizing its potential to address the unmet need in treating hepatorenal syndrome. This designation is significant as it provides the drug with various incentives, such as market exclusivity and accelerated development timelines, to help bring this potentially life-saving therapy to market faster.
AptaBio Therapeutics’ APX-115
Phase – II
APX-115, developed by AptaBio Therapeutics, is a potent small-molecule inhibitor of NADPH-oxidase (NOX) isozymes. These enzymes catalyze the NADPH-dependent generation of superoxide and other reactive oxygen species (ROS), which can lead to tissue damage and disruption of cellular functions. By inhibiting NOX isozymes, APX-115 aims to reduce oxidative stress and prevent the damage associated with conditions like contrast-induced acute kidney injury (CI-AKI).
In January 2023, the U.S. FDA approved the Phase II clinical trial plan for APX-115 to evaluate its efficacy in treating CI-AKI. The trial, which began patient recruitment in October 2023, aims to assess how APX-115 can mitigate the kidney damage caused by the use of contrast agents in medical imaging procedures. The trial aims to conclude by the end of 2024, potentially paving the way for further drug development to treat CI-AKI.
Future Outlook for Acute Kidney Injury Treatments
The treatment landscape for acute kidney injury continues to face significant challenges, especially in developing curative and disease-modifying therapies. While supportive care, such as dialysis and fluid management, remains essential, there is a critical need for treatments that can directly target the underlying causes of AKI and prevent long-term kidney damage. Addressing AKI in high-risk patients, such as those undergoing major surgeries or suffering from sepsis, is a key area where improvements are necessary to reduce mortality and enhance recovery.
Encouragingly, a strong pipeline of innovative therapies is under development, with many focusing on targeting the mechanisms of injury in AKI, such as oxidative stress, inflammation, and renal cell damage. These therapies aim to not only prevent AKI progression but also promote kidney recovery, offering the potential for disease-modifying treatments. Additionally, regenerative approaches like stem cell therapies and novel bioengineered solutions are advancing, holding promise for restoring kidney function and reversing some of the damage caused by AKI.
Ongoing research aims to develop new treatments that target AKI across various stages, from preventing the condition in high-risk patients to aiding recovery in those with established kidney injury. With an increasing understanding of the mechanisms behind AKI and its impact on renal function, the future outlook for AKI therapies is optimistic. In the next decade, transformative treatments will likely emerge, offering new avenues for managing AKI and significantly improving patient outcomes, quality of life, and reducing the long-term burden of kidney disease.

Downloads
Article in PDF
Recent Articles
- Notizia
- Tecelra by Adaptimmune: First FDA-Approved Engineered Cell Therapy for Solid Tumors; GSK’s ...
- FDA just lost a gem as Scott Gottlieb resigns
- Madrigal Wins EU Approval for REZDIFFRA in MASH With Liver Fibrosis; Valneva Faces FDA License Su...
- Solis Mammography’s Acquisition of MUSIC Imaging Center; Nikon’s SI-PH Phase Condenser Accessory ...



