CAR T-Cell Therapies in Non-Hodgkin’s Lymphoma Treatment: A Revolutionary Approach
Sep 02, 2024
Table of Contents
CAR-T cells offer a lasting benefit through a single treatment, sparing patients with high-risk conditions from the toxicity associated with salvage chemotherapy and autologous transplants. These approvals have changed the standard of care for patients who are either resistant to initial treatment or experience early recurrence after first-line therapy.
As per DelveInsight’s estimates, in 2023, the total eligible cases of NHL for CAR-T were around 126K in the 7MM. In 2023, the total NHL incident cases in selected subtypes were around 17K cases in Germany. DLBCL stands out as the most prevalent subtype among selected types of non-Hodgkin lymphoma in EU4 and UK, with approximately 32K cases reported in 2023.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Iovance’s Breakthrough: Amtagvi Paves the Way for Cell Therapies in Solid Tumors
- Bispecific and Trispecific Antibodies: Are They Better Than CAR-Ts?
- CAR-T in killing tumors; Amunix raises $73M; Autism findings in protein-targeted treatment
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
- The Changing Landscape of Multiple Myeloma Therapies Market
CAR-T cell therapy has revolutionized the treatment of aggressive lymphomas like DLBCL and is also now an option for R/R follicular lymphoma, although follow-up remains short. The treatment landscape for NHL is multifaceted and dependent on various factors including the subtype of NHL, disease stage, overall health, and patient preferences. CAR-T cell therapy has emerged as a promising avenue within this landscape, particularly for aggressive B-cell NHLs that have proven refractory to standard therapies.

Approved CAR-Ts for Non-Hodgkin’s Lymphoma Treatment
The primary treatment options for hematological malignancies currently include stem cell transplantation, chemotherapy, and radiotherapy. As our understanding of the molecular genetics underlying these diseases has grown, new immunotherapy techniques have emerged as a promising treatment avenue. While immunotherapy was once considered a potentially beneficial option, it has now become an established cancer treatment that has transformed the field of cancer therapy over the last decade.
One of the most promising immunotherapy strategies is CAR-T cell therapy, which has shown high effectiveness in treating hematological cancers. CAR-T cells have been predominantly approved for R/R cases in various NHL subtypes, including DLBCL, mantle cell lymphoma, follicular lymphoma, and marginal zone lymphoma, supported by Phase II studies. Recently, Phase III data in DLBCL after early relapse following initial treatment have also contributed to this approval. Currently, four CAR-T therapies are approved for NHL: YESCARTA, KYMRIAH, BREYANZI, and TECARTUS.
Gilead Sciences’ YESCARTA
YESCARTA (axicabtagene ciloleucel) is an autologous T-cell immunotherapy that is genetically modified to target CD19. The US FDA initially approved YESCARTA in October 2017 for treating adult patients with specific types of large B-cell lymphoma. YESCARTA is the only CAR-T being evaluated in 1L DLBCL. In March 2021, YESCARTA received accelerated approval from the FDA for use in adult patients with relapsed or refractory follicular lymphoma after failing two or more systemic therapies. By April 2022, the FDA expanded its approval to include adult patients with large B-cell lymphoma that is resistant to or relapses within 12 months of first-line chemoimmunotherapy.
Novartis’ KYMRIAH
KYMRIAH (tisagenlecleucel) is a CD19-targeted, genetically engineered autologous T-cell therapy that uses a lentiviral vector to modify T cells to express an anti-CD19 CAR. In May 2018, the US FDA approved KYMRIAH for adult patients with R/R large B-cell lymphoma after at least two prior systemic treatments, including DLBCL not otherwise specified, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. In May 2022, the FDA granted accelerated approval to KYMRIAH for adult patients with R/R follicular lymphoma following two or more prior lines of systemic therapy.
Bristol Myers Squibb’s BREYANZI
BREYANZI is a CAR-T cell therapy aimed at targeting CD19, a surface glycoprotein involved in normal B-cell development. In February 2021, the US FDA approved BREYANZI for treating adult patients with specific types of large B-cell lymphoma who have either not responded to or have relapsed after at least two other systemic treatments. In April 2022, Bristol Myers Squibb announced that the European Commission granted marketing authorization for BREYANZI to treat adult patients with R/R DLBCL, PMBCL, and follicular lymphoma grade 3B after two or more lines of systemic therapy.
On 14 March 2024, Bristol Myers Squibb announced that the US FDA approved BREYANZI as the first and only CAR T-cell therapy for adults with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma.
Gilead Sciences’ TECARTUS
TECARTUS is a genetically engineered autologous T-cell immunotherapy that targets CD19. It binds to cancer cells and normal B cells that express CD19. Research has shown that when anti-CD19 CAR T cells interact with CD19-positive target cells, the CD28 and CD3-zeta costimulatory domains trigger signaling pathways that result in T cell activation, proliferation, the development of effector functions, and the release of inflammatory cytokines and chemokines. This process ultimately leads to the destruction of CD19-positive cells. TECARTUS is approved for the treatment of adult patients with R/R MCL. TECARTUS stands as the sole CD-19 CAR-T therapy approved for use in 3L+ MCL treatment.
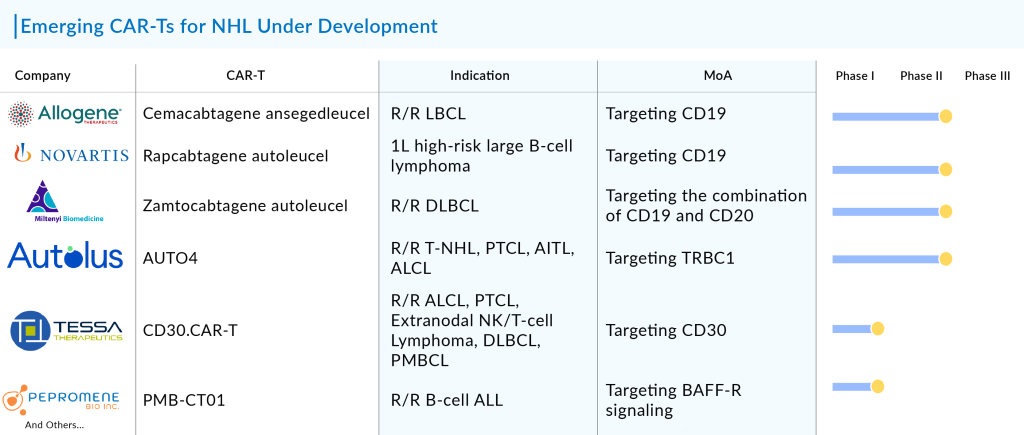
Promising CAR-Ts for NHL Treatment on the Horizon
The developing CAR-T therapies for NHL encompass drugs at various stages—late, mid, and early—in different treatment lines and indications. These therapies primarily target B-cell lymphomas like DLBCL, FL, MCL, MZL, and CLL/SLL, among others, with one also aimed at T-cell lymphoma, specifically PTCL.
The majority of advancements in the NHL sector focus on DLBCL within the CAR-T pipeline. The landscape of pipeline activity in DLBCL appears robust, characterized by the involvement of numerous entities such as Allogene Therapeutics, Miltenyi Biomedicine, CRISPR Therapeutics, 2seventy bio, and others, who are actively assessing their CAR-T therapies in the third-line setting. Concurrently, established CAR-T therapies are advancing into earlier lines of treatment.
Although YESCARTA has received approval for various types of DLBCL, it is not approved for first-line treatment or for patients with second-line or later non-transplant eligible cases in the United States. As a result, the company is running trials to address these areas. In the evolving DLBCL treatment landscape, several CAR-T therapies, including zamtocabtagene autoleucel, ALLO-501A, YTB323, and others, are currently undergoing trials in different phases and targeting various patient groups.
In the space of indolent NHL, the MZL subtype is drawing increasing interest. Trials for both YESCARTA and BREYANZI are in progress for MZL, similar to those conducted for follicular lymphoma. YESCARTA has already been approved for follicular lymphoma, and BREYANZI is expected to gain approval shortly based on comparable trial results. Currently, there is no available data for BREYANZI and YESCARTA regarding MZL. Once both CAR-T therapies are approved for MZL, they are likely to alter the treatment landscape for this condition significantly.
At present, only autologous CAR-T therapies have received approval, but the development pipeline is mainly focused on these, with an increasing number of allogeneic CAR-T therapies also being explored. Companies working on allogeneic CAR-T cell therapies include Allogene Therapeutics (ALLO-501A), Wugen (WU-CART-007), Imugene (PBCAR0191), and others.
Cemacabtagene ansegedleucel (ALLO-501A): Allogene Therapeutics
Cemacabtagene ansegedleucel (ALLO-501A), an advanced anti-CD19 AlloCAR-T therapy, is designed without the rituximab recognition domains present in ALLO-501. This modification may enable its use in a wider range of patients, including those with NHL who have recently been exposed to rituximab. ALLO-501A utilizes Cellectis TALEN technology. The company is performing long-term follow-up in a Phase I clinical trial (ALPHA trial) for ALLO-501 in patients with relapsed or refractory NHL. Additionally, a Phase I/II pivotal trial for ALLO-501A (ALPHA2 trial) was initiated in the second quarter of 2020.
In June 2022, Allogene announced that the US FDA granted RMAT designation to cemacabtagene ansegedleucel for R/R LBCL. In 2021, the FDA also awarded FTD status to cemacabtagene ansegedleucel for treating adult patients with R/R DLBCL.
Allogene’s latest presentation indicates that the preliminary data for the Phase I ALPHA2 trial in R/R CLL is expected in 2024. Patient enrollment is still in progress. Additionally, preparations are ongoing for the pivotal ALPHA3 first-line (1L) consolidation trial in LBCL.
WU-CART-007: Wugen
WU-CART-007 is an allogeneic, off-the-shelf, fratricide-resistant CAR-T cell therapy targeting CD7, designed to address the challenges of using CAR-T cells for treating CD7+ blood cancers. To avoid fratricide and reduce the risk of (GvHD, Wugen utilizes CRISPR/Cas9 gene editing to remove CD7 and the T-cell receptor alpha constant (TRAC). This therapy is produced from healthy donor T-cells, thereby avoiding the potential for malignant cell contamination seen with autologous CAR-T therapies. WU-CART-007 is currently under investigation in a global Phase I/II clinical trial for relapsed or refractory T-ALL and LBL.
WU-CART-007 has been granted Orphan Drug, Fast Track, Rare Pediatric Disease, and Regenerative Medicine Advanced Therapy (RMAT) designations by the FDA, and it has also received PRIME (“Priority Medicines”) status from the European Union for treating R/R T-ALL and T-LBL.
PBCAR0191: Imugene
Azer-cel is a promising candidate for a first-in-class allogeneic CAR T therapy targeting CD19+ for patients with relapsed CAR T. It has shown encouraging results in patients with DLBCL who have relapsed after CAR T therapy, with high overall response rates and molecular remissions. The Phase Ib data suggest that Azer-cel could enhance outcomes for this expanding patient group with significant unmet needs.
CAR T-Cell Therapies in NHL: Market Trends and Outlook
The landscape for CAR T-cell therapy in the NHL market is set to evolve significantly in the coming years. The sector is marked by a rise in research and development as pharmaceutical companies compete to introduce new treatments. With multiple CAR T-cell therapies gaining FDA approval, the competition among producers is fierce, fostering innovation and increased investment. Consequently, the field is characterized by a vibrant environment of ongoing clinical trials, collaborations, and strategic alliances, all focused on enhancing the scope and effectiveness of CAR T-cell therapies for NHL.
As per DelveInsight analysis, the total market size for CAR-T in NHL in the United States was around USD 1.2 billion in 2023, expected to rise by 2034. Approximately 90% of the overall CAR-T market is projected to be dominated by Aggressive B-cell NHL, with the remaining 10% attributed to indolent B-cell NHL.
BREYANZI and YESCARTA are expected to dominate the CAR-T in the NHL market in the coming years. YESCARTA appears to have the upper hand in the battle for CAR-T supremacy, given it has been in the market before BREYANZI. Currently, YESCARTA captures the highest patient share among all CAR-T therapies.

It is anticipated that KYMRIAH’s revenue growth will face challenges in the 3L+ DLBCL market across the 7MM, primarily due to intense competition from Gilead’s YESCARTA and Bristol Myers Squibb’s BREYANZI in this segment. Additionally, setbacks stemming from trial failures in the 2L+ DLBCL space further compound this outlook. However, KYMRIAH’s revenue is projected to see an upward trajectory in emerging markets and in the treatment of follicular lymphoma.
CAR T-Cell Therapies in NHL: What Lied Ahead?
CAR T-cell therapies represent a groundbreaking advancement in the treatment of NHL, offering new hope for patients who have exhausted traditional options. The future of CAR T-cell therapies in NHL is promising, with ongoing research focused on enhancing the efficacy and safety of these treatments. Emerging strategies include the development of next-generation CAR T-cells that target multiple antigens simultaneously or employ novel co-stimulatory domains to improve persistence and reduce relapse rates. Clinical trials are exploring these advanced approaches, which aim to provide more durable remissions and broaden the applicability of CAR T-cell therapy to a wider range of NHL subtypes.
Additionally, efforts are being made to address the challenges associated with CAR T-cell therapy, such as managing side effects and improving accessibility. Innovations in cell manufacturing and administration processes, as well as strategies to mitigate cytokine release syndrome and neurotoxicity, are key areas of focus. As these advancements progress, CAR T-cell therapies are likely to become a more integral part of NHL treatment regimens, offering personalized and targeted options that could significantly improve patient outcomes and transform the landscape of lymphoma care.

Downloads
Article in PDF
Recent Articles
- Prolong’s PP-007 Fast-Tracked for Stroke; FDA Expands JYLAMVO Pediatric Approval; Corcept’s Cushi...
- Novartis gets CAR-T drug; Shire aims to block; Xeljanz stands; Payer snubs; Lawmakers pass a bill
- 66 Year Ohio Woman Becomes Cancer Free, Thanks to CAR T Cell Therapy
- Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
- Future Perspectives Of CAR-T Cell Therapy Market In Acute Lymphoblastic Leukemia (ALL)