The Future of Parkinson’s Disease Treatment: Unlocking the Potential of Cell and Gene Therapy
Nov 18, 2024
Table of Contents
Parkinson’s disease is the second most common neurodegenerative disorder after Alzheimer’s disease. It affects about 10 million people worldwide. The average age of onset for Parkinson’s disease is around 60; however, approximately 10-15% of patients experience early-onset PD before the age of 50.
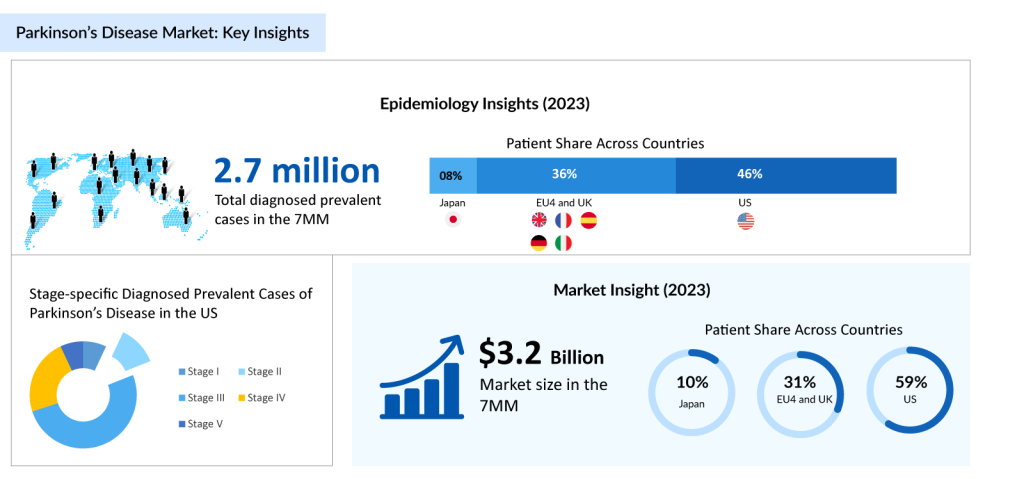
In 2023, there were 2.7 million diagnosed prevalent cases of Parkinson’s disease in the 7MM. Of these, the United States accounted for 45% of the cases, while Japan and Germany represented 9% and 18% of the cases, respectively, according to DelveInsight’s estimates.
Downloads
Article in PDF
Recent Articles
- Toujeo to treat Type 1 Diabetes; TauRx for Alzheimer’s patients
- 10 Game-Changing Acute Myeloid Leukemia Drugs Revolutionizing Treatment
- Daiichi Sankyo’s Ezharmia; Pfizer & Sangamo Hemophilia A Gene Therapy Trial; Approval for Fe...
- Japan has got new ways to treat corneas
- Parkinson’s Disease: How Close are we to a Cure?
As per the estimates, the gender distribution of the disease suggests a male predominance, with approximately 54% male and 46% female cases in the 7MM in 2023. Among the stage-specific diagnosed prevalent cases of Parkinson’s disease in Japan, the highest cases were contributed by Stage III in 2023. Currently, there’s no cure for Parkinson’s, but treatments like medication, lifestyle changes, and deep brain stimulation can manage symptoms and improve quality of life.
To grab more knowledge on Parkinson’s disease, visit our blog “Evaluation of Rapidly Evolving Parkinson’s Disease Therapeutic Market”
Persistent Gaps in Parkinson’s Disease Treatment
Current Parkinson’s disease treatments, including levodopa and dopamine agonists, mainly focus on managing symptoms rather than addressing the root cause of neuronal degeneration. As a result, their effectiveness declines over time, leading to issues such as motor fluctuations, dyskinesias, and non-motor symptoms, which significantly affect patients’ quality of life. While several approved therapies target motor symptoms and enhance movement in Parkinson’s disease patients, the US FDA recently approved CREXONT extended-release capsules in August 2024 for the treatment of Parkinson’s disease.
CREXONT is a novel oral formulation of carbidopa/levodopa (CD/LD) that combines immediate-release (IR) granules and extended-release (ER) pellets. This medication is prescribed for the treatment of Parkinson’s disease, Parkinson’s disease caused by brain infections or inflammation, and Parkinson’s-like symptoms resulting from carbon monoxide or manganese poisoning in adults. The ER pellets in CREXONT contain levodopa coated with a sustained-release polymer for gradual drug release, a mucoadhesive polymer to help the granules stay at the absorption site longer, and an enteric coating to prevent early breakdown in the stomach. This formulation is different from RYTARY, an extended-release CD/LD treatment for Parkinson’s disease approved by the US FDA in 2015.
Despite limited major advancements in Parkinson’s disease treatment since levodopa’s introduction, several unmet needs persist. The current Parkinson’s disease market lacks curative or disease-modifying therapies, offering only symptomatic treatment through a multidisciplinary approach. Although medication and neurosurgical techniques have progressed over the decades, a significant gap remains in effectively managing motor symptoms. Improved control over tremors, gait and balance, posture, dexterity, and communication skills remain a major therapeutic challenge. Additionally, addressing psychosis in Parkinson’s patients is another critical unmet need.
How Cell and Gene Therapies Are Changing Parkinson’s Care?
Cell and gene therapies are revolutionizing the landscape of Parkinson’s care, offering hope to patients who once faced limited options. Traditional treatments focus on managing symptoms, but these innovative therapies aim to tackle the root causes of the disease. By introducing healthy cells or correcting faulty genes, scientists are developing groundbreaking solutions that could slow, halt, or even reverse the progression of Parkinson’s. This new era of precision medicine is pushing the boundaries of what was once thought possible, offering a glimpse of a future where Parkinson’s no longer dictates patients’ lives.
With advancements in gene editing and cell-based treatments, Parkinson’s therapies are becoming increasingly tailored to individual patients. Cell therapies, like stem cell-based treatments, aim to regenerate the brain cells lost to the disease, while gene therapies focus on repairing or replacing malfunctioning genes that contribute to neurodegeneration. This personalized approach holds the promise of more effective, long-lasting treatments that address the unique genetic and biological factors at play in each patient, paving the way for targeted solutions that could transform Parkinson’s care.
Emerging Cell and Gene Therapies Shaping the Future of Parkinson’s Disease Treatment
Cell and gene therapies are paving the way for groundbreaking treatments for Parkinson’s disease, offering hope where traditional therapies have fallen short. By targeting the root causes of Parkinson’s disease at the genetic and cellular levels, these innovative approaches aim to slow, stop, or even reverse the progression of this debilitating neurodegenerative disease. Advances in gene editing technologies like CRISPR, paired with regenerative cell therapies, are unlocking the potential to repair or replace damaged neurons, potentially restoring motor function and improving the quality of life for patients. The future of PD treatment is no longer just about symptom management, but about tackling the disease at its core.
The potential of cell and gene therapies for Parkinson’s is already being tested in clinical trials, with promising early results. As these therapies progress from the lab to the clinic, they’re shifting the narrative around Parkinson’s care from merely symptom management to actual disease modification. Patients participating in trials are already experiencing improvements in motor function and quality of life, sparking excitement among both clinicians and researchers.
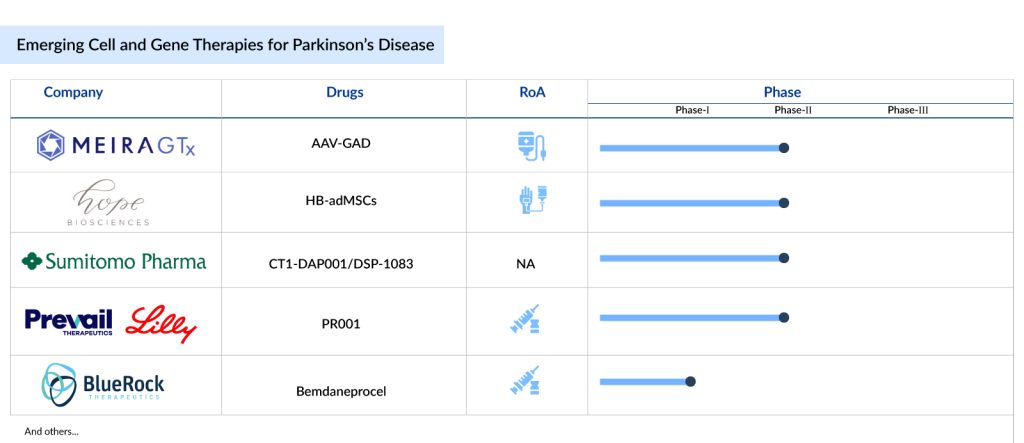
The cell and gene therapy in the Parkinson’s disease market has a promising outlook. The current emerging cell and gene therapy in the Parkinson’s disease pipeline is robust; with various therapies being developed, some of which include MaavRx’s gene therapy, MeiraGTx’s AAV-GAD, Hope Biosciences’ HB-adMSCs, Sumitomo Pharma’s CT1-DAP001/DSP-1083, Prevail Therapeutics/Eli Lilly’s PR001 (LY3884961), and BlueRock Therapeutics’ Bemdaneprocel (BRT-DA01), among others.
Apart from cell and gene therapies, a wide range of medications is currently being developed, including those targeting alpha-synuclein pathology, which is widely regarded as a key factor in the neurodegeneration associated with Parkinson’s disease. These treatments hold promise as potential disease-modifying therapies in the near to medium-term future. Coupled with various regenerative approaches, such as stem cell and gene therapies, advancements in Parkinson’s disease treatments are expected in the coming years, with several new, effective options likely to emerge.
The emerging Parkinson’s disease pipeline is promising, with several late-stage drugs anticipated to enter the Parkinson’s disease market in the coming years. These include Supernus Pharmaceuticals/Britannia Pharmaceuticals’ SPN-830 (apomorphine infusion pump), AbbVie’s tavapadon, Pharma Two B’s P2B001 (extended-release pramipexole and rasagiline), and Mitsubishi Tanabe Pharma Corporation (NeuroDerm)’s ND0612 (levodopa/carbidopa), among others. The approval of these therapies could have a significant impact on market dynamics, though their success rates remain uncertain.
To know more about emerging Parkinson’s disease therapies, read our blog “Most Promising Therapies in the Parkinson’s Disease Treatment Market”
With continued advancements, we’re on the cusp of a major breakthrough that could provide a lasting, transformative impact on Parkinson’s treatment, offering renewed hope for those living with the condition.
The Road Ahead: Challenges and Opportunities in Parkinson’s Disease Cell and Gene Therapies
Parkinson’s disease remains one of the most debilitating neurological disorders, with current treatments only addressing symptoms rather than halting or reversing disease progression. The future of Parkinson’s disease therapy lies in groundbreaking cell and gene therapies, which have shown tremendous potential in preclinical and early clinical trials. However, the road ahead is not without significant challenges. Overcoming the complexities of delivering therapeutic genes or cells to targeted regions of the brain, navigating the regulatory landscape, and ensuring long-term safety and efficacy are hurdles that researchers and clinicians must clear to make these therapies a reality for patients.
On the flip side, the opportunities for cell and gene therapies in Parkinson’s disease are immense. Advancements in gene editing technologies like CRISPR, and the increasing understanding of the molecular mechanisms driving Parkinson’s, are paving the way for more personalized and targeted treatments. By potentially correcting the genetic mutations causing Parkinson’s disease or delivering neuroprotective genes, these therapies could significantly improve patients’ quality of life and even delay disease onset. Moreover, with collaborations between biotech companies, research institutions, and advocacy groups, there is a concerted effort to address both the scientific and logistical challenges to move these therapies into the clinic.
As the field progresses, new innovations and discoveries may dramatically reshape our approach to treating Parkinson’s disease. With the right investment, collaboration, and clinical trials, cell and gene therapies have the potential to offer hope where conventional treatments fall short. The journey toward effective and accessible therapies for Parkinson’s patients is challenging but brimming with transformative possibilities, making it one of the most exciting areas in neurodegenerative disease research today.