Cervical Cancer: Current Scenario
Nov 08, 2024
Cervical cancer remains a leading cause of death among women worldwide. While increased awareness and early diagnosis have significantly reduced fatalities over the years, the risk is still substantial. A major factor in the development of cervical cancer is persistent infection with Human Papillomavirus (HPV), which is linked to the vast majority of cases. This preventable virus underscores the importance of screening and vaccination efforts. While strides have been made in managing cervical cancer, ongoing vigilance, education, and access to preventative care are crucial in reducing its impact even further.
Research showed that psychosocial stress is one of the crucial contributors to the prolonged infection of HPV. Women who smoke, take drugs, etc., are more prone to HPV infection, which further increases the chances of developing cervical cancer. Women suffering from Systemic Lupus Erythematosus (SLE) and inflammatory bowel Syndrome (IBD) like Crohn’s disease should take extra care as they can be more likely to develop cervical cancer. Age is also an important factor. Trends indicate that the occurrence of cervical cancer rises between the late teens and mid-30s. Women with lowered immunity are at high risk of developing this cancer. Other factors, such as multiple pregnancies, oral contraceptives, more than one sex partner, etc., are more likely to contribute to the occurrence of cervical cancer.
Since cervical cancer has a large scope for treatment if detected in early stages, researchers are focusing on developing better ways of detecting precancer and cervical cancer. Prevention has always been better than cure, and in the case of cancer, early diagnosis increases the survival rate. Regular screening (Pap test and HPV Test) and vaccination are suggested for prevention. The treatment is based on the use of surgery, Chemotherapy, Radiation therapy, and targeted therapy.
Downloads
Article in PDF
Recent Articles
- Global Hemophilia Market
- FDA Grants Fast Track for Lin BioScience’s LBS-007; Alnylam’s AMVUTTRA sNDA Under Review; FDA App...
- December 7 Business Cocktail
- Breakthrough Success: AUGMENT-101 Trial Hits Primary Endpoint with Consistent CR/CRh Rates Across...
- Vasomotor Symptoms of Menopause-Emerging Therapies
Cervical cancer remains a significant health issue worldwide, especially in low- and middle-income countries, where limited access to screening and treatment contributes to higher incidence and mortality rates. Despite awareness initiatives and advancements in preventive measures like the HPV vaccine, thousands of women continue to be diagnosed with cervical cancer every year. For those facing a diagnosis, the choice of cervical cancer treatment often depends on factors such as the cancer’s stage, the patient’s health, and personal preferences. Early diagnosis is crucial, as it allows for more localized treatment options with a higher chance of curative success.
Traditionally, surgery and radiation therapy have been the mainstays of early-stage cervical cancer treatment. Surgical options may range from local excision to more extensive procedures like hysterectomy, depending on the cancer’s spread. Radiation therapy, either external or internal (brachytherapy), is also commonly used and can be curative in the early stages.
However, as the cancer progresses, treatment often needs to be intensified with combinations of radiation and chemotherapy to manage larger or more invasive tumors. For example, chemoradiotherapy (CRT) combines radiation with drugs to increase the cancer-killing effect, though it comes with potential long-term side effects like organ damage, impacting the patient’s quality of life.
In cases of persistent, recurrent, or metastatic, cervical cancer drugs like chemotherapy doublets (e.g., cisplatin and paclitaxel) are often used as a standard approach. While these chemotherapy agents are effective, their impact is somewhat limited by adverse effects and resistance over time. Recognizing these limitations, researchers have focused on incorporating targeted therapies, which promise a more precise approach.
In 2014, the FDA approved the use of Avastin (bevacizumab), a monoclonal antibody targeting the VEGF pathway, for use with chemotherapy in advanced cervical cancer. This combination marked a significant shift in the treatment paradigm by offering patients with metastatic disease a new line of defense.
More recently, the rise of immunotherapies has expanded treatment options in a revolutionary way. KEYTRUDA (pembrolizumab), for instance, was the first immune checkpoint inhibitor approved for cervical cancer. This drug works by blocking the PD-1 pathway, which cancer cells use to evade detection by the immune system. By inhibiting PD-1, KEYTRUDA enhances the immune system’s ability to recognize and attack cancer cells.
The FDA approved it in 2024 for use with chemoradiotherapy for certain advanced stages of cervical cancer, solidifying immunotherapy’s role in the market. The European Commission and Japan’s Ministry of Health have also approved KEYTRUDA in combination with chemotherapy, with or without bevacizumab, highlighting its global reach in advanced treatment strategies.
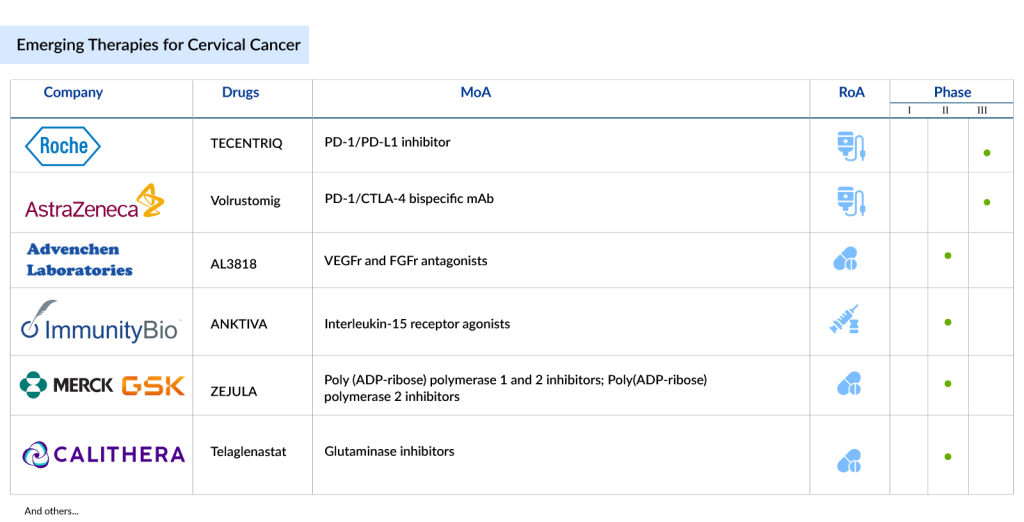
In addition to approved therapies, there is an active cervical cancer pipeline of emerging drugs with novel mechanisms of action. Among these is TIVDAK (tisotumab vedotin-tftv), developed by Genmab and Pfizer, which received accelerated FDA approval in 2021 for recurrent or metastatic cervical cancer. TIVDAK is an antibody-drug conjugate that delivers a cytotoxic agent directly to cancer cells by targeting tissue factor (TF), a protein commonly overexpressed in cervical cancer. This selective delivery minimizes damage to surrounding healthy cells, offering a promising option for patients who have exhausted prior treatments.
Further in the pipeline is AstraZeneca’s Volrustomig, a bispecific monoclonal antibody targeting PD-1 and CTLA4. Currently, in Phase III trials, Volrustomig is designed to treat high-risk, locally advanced cervical cancer and shows potential as a next-generation immunotherapy with a dual mechanism of immune activation.
Similarly, Precigen’s PRGN-2009, an experimental therapy based on gorilla adenovirus technology, aims to treat HPV-associated cancers by enhancing the immune response against cancer cells. This unique approach allows for repeated dosing without increasing neutralizing antibodies, which could be a game-changer in cervical cancer therapies.
The current scenario in the cervical cancer market is thus dynamic and rapidly evolving. With the advent of biosimilars like Amgen’s MVASI, a counterpart to Roche’s Avastin, patients have access to cost-effective options that make biologics more affordable. This development is particularly beneficial in regions with budget constraints, supporting a more equitable healthcare landscape. Biosimilars offer the same efficacy as their original counterparts but at a reduced cost, enabling broader access to life-saving treatments. Several other companies are working on creating therapies for the treatment of the disease.

Today, the landscape for women living with cervical cancer looks more promising than ever. The focus on precision medicine, including immune checkpoint inhibitors, antibody-drug conjugates, and biosimilars, represents a paradigm shift in cancer treatment. For patients facing recurrent or advanced disease, these therapies offer new hope and, in some cases, significantly extend survival. As more innovative drugs progress through clinical trials, the cervical cancer pipeline is expected to diversify further, with therapies tailored to meet the unique needs of patients across different stages of disease and genetic backgrounds. With ongoing advancements, the future of cervical cancer treatment is steadily moving toward personalized and more effective options, aiming to improve survival rates and quality of life for countless women worldwide.

Downloads
Article in PDF
Recent Articles
- Notizia
- Bile Duct Cancer (Cholangiocarcinoma) -Pipeline Insights, 2015
- Bayer Phase III NSCLC Trial; D&D Pharmatech Gets FDA Nod for GLP-1R Agonist in Multiple Sclet...
- Inozyme Raises $67M; Shanghai Miracogen partners with Synaffix; EdiGENE raises $15M; Antengene an...
- A promising immune-stimulating target



