Cervical Cancer: What You Need to Know!
Nov 12, 2024
Table of Contents
No woman should ever lose her life to cervical cancer, yet too many still do. This devastating disease strikes at the cervix—the gateway to the womb, the very place where new life begins. Primarily affecting women between the ages of 30 and 45, it silently preys on those who are sexually active. The cervix, a small but vital part of the uterus, holds the promise of motherhood, yet for many, it becomes the battleground where a silent and dangerous enemy lurks. Cervical cancer, though preventable and treatable, continues to claim lives, highlighting a tragic gap in awareness and care.
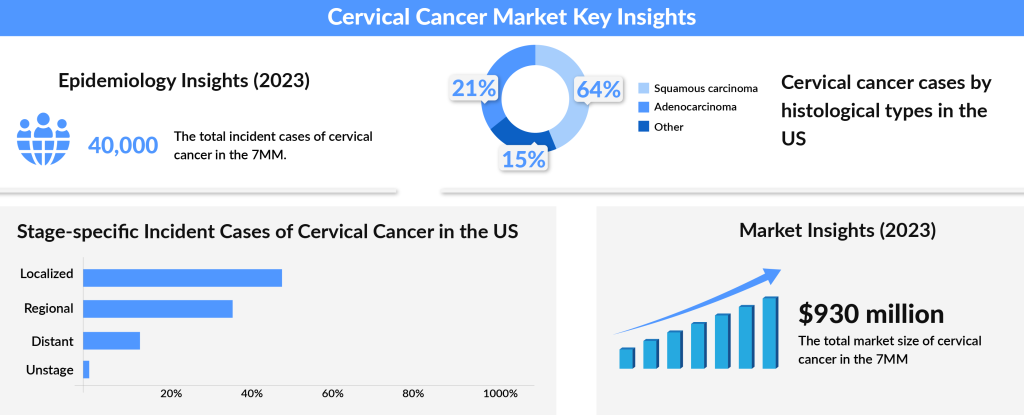
Cervical cancer, one of the most preventable cancers, is primarily caused by a persistent infection with human papillomavirus (HPV). As per DelveInsight analysis, the total incident cases of cervical cancer in the 7MM comprised 40K in 2023 and are projected to decrease by 2034. The US contributed to the largest incident population of cervical cancer, accounting for nearly 34% of the 7MM in 2023. While most HPV infections are harmless and clear on their own, certain strains, particularly type 16, can silently wreak havoc. When this virus lingers, it can lead to cancer not only in the cervix but also in the vulva, vagina, anus, penis, and even the oropharynx. The virus targets epithelial cells—those that line our body surfaces—and disrupts normal cell growth, causing abnormal cell division and genetic damage, which may eventually lead to cancer.
Downloads
Click Here To Get the Article in PDF
Recent Articles
Cervical cancer continues to be a major global health challenge, particularly in low- and middle-income countries where access to screening and treatment is often limited. This disparity contributes to higher rates of both diagnosis and mortality. Despite progress in raising awareness and the introduction of preventive measures like the HPV vaccine, thousands of women are still diagnosed with cervical cancer each year. For those confronted with a diagnosis, the cervical cancer treatment options depend on factors such as the stage of the cancer, the patient’s overall health, and individual preferences. Early detection plays a critical role, as it offers the best opportunity for localized treatments, which are more likely to lead to a successful cure.
Global Disparities in Prevention and Treatment
Fortunately, medical science offers us powerful tools to combat this threat. Two effective vaccines targeting the high-risk HPV types—16 and 18, as well as types 16, 18, 6, and 11—have been introduced in many developed countries as a preventive measure, offering protection against the most cancer-causing strains of the virus. HPV testing has also become a crucial part of secondary prevention, helping detect early signs of infection and enabling better treatment outcomes. In fact, HPV testing is far more sensitive than traditional cytology (Pap smears), which could make it possible to extend screening intervals, reducing unnecessary tests while improving early detection.
Despite these advancements, cervical cancer remains a pressing issue, particularly in developing countries. If prevention strategies, like vaccination and HPV testing, could be widely implemented in these regions, thousands of lives could be saved.
The virus spreads through sexual contact, but many women’s immune systems are able to fight off the infection before it causes harm. However, certain factors can increase the risk, including smoking, having multiple children, long-term use of birth control pills, and a weakened immune system, such as in those with HIV. While the majority of HPV infections never lead to cancer, for those that do, prevention through vaccination and early detection is key. By empowering women with knowledge and access to these life-saving strategies, we can reduce the global burden of cervical cancer and ensure that no woman has to face this preventable tragedy.
Cervical cancer treatment has evolved significantly in recent years, driven by advances in immunotherapy, targeted therapies, and antibody-drug conjugates (ADCs). For patients with recurrent or metastatic cervical cancer (r/mCC), TIVDAK (tisotumab vedotin-tftv) has emerged as a significant breakthrough. This FDA-approved ADC targets tissue factor (TF) on cancer cells, delivering a potent cytotoxic agent directly to the tumor. The approval of TIVDAK in 2024, based on positive Phase III clinical trial results, marks a major milestone in cervical cancer therapy. Traditionally, chemotherapy doublets, such as cisplatin-paclitaxel, have been the standard, but recent combinations like cisplatin-paclitaxel with bevacizumab (AVASTIN) have further enhanced survival outcomes. Immunotherapies like KEYTRUDA (pembrolizumab) and LIBTAYO (cemiplimab) are now integral parts of treatment, boosting immune response and significantly improving progression-free survival (PFS) and overall survival (OS) in advanced cases of cervical cancer.
The cervical cancer market is poised for significant growth, with an anticipated increase in market size from approximately USD 930 million in 2023 to a much larger figure by 2034. The expansion of immunotherapy, biologic agents, and targeted therapies is expected to transform the treatment landscape. With emerging therapies like Lifileucel, a one-time, personalized T-cell therapy, and PRGN-2009 showing great promise, the future of cervical cancer treatment looks brighter than ever. By incorporating innovative treatment regimens and personalized approaches, these therapies will continue to drive improvements in survival rates and offer new hope for patients with cervical cancer. As the landscape shifts, the incorporation of biomarker-driven treatments and combination therapies will be critical in achieving better patient outcomes.
The Role of HPV Vaccination and Screening
According to WHO, around 660,000 new cases and 350,000 deaths were attributed to cervical cancer worldwide in 2022, with approximately 80% occurring in low- and middle-income countries. Effective diagnostic methods and the widespread availability of HPV vaccines in developed countries have successfully curbed incidence rates, while limited healthcare infrastructure in developing nations continues to present significant challenges. Cervical cancer’s primary cause, human papillomavirus (HPV), is a sexually transmitted virus responsible for nearly all cases. To combat this, screening methods such as Pap smears and HPV DNA tests play a critical role in early detection, significantly improving survival rates.
For young, sexually active women, screening every three years from age 21, or every five years with HPV co-testing from age 30, is recommended.
HPV vaccines like GARDASIL 9, CERVARIX, and GARDASIL have demonstrated near-total efficacy against high-risk HPV types, offering protection for 6-10 years and contributing to a marked decline in HPV infections, cervical cancer cases, and related conditions such as anogenital warts in vaccinated populations. Vaccination programs across the US, UK, and Germany now also include young males, expanding immunity and decreasing HPV transmission. Despite some controversies, such as high costs and concerns around early-age vaccination, these preventive measures have shown immense success. Increasing awareness about the benefits of HPV vaccination, especially in developing countries, remains crucial for cervical cancer prevention.
With continued advocacy, education, and accessible healthcare solutions, we are on the cusp of a future where cervical cancer can be significantly diminished, potentially saving countless lives and creating a legacy of proactive cancer prevention.
The widespread use of the HPV vaccine has significantly reduced cervical cancer and other HPV-related cancers, as well as precancerous cervical lesions, which are key indicators of future cancer risk. With high vaccination rates, the global burden of cervical cancer continues to decline.
Research is also advancing in therapeutic vaccines, with promising candidates like Nykode Therapeutics’ VB10.16 and ISA Pharmaceuticals’ ISA101b showing potential for treating existing HPV-related cancers. The World Health Organization’s efforts to eliminate cervical cancer are gaining momentum, with HPV vaccines estimated to prevent up to 70% of cervical cancer cases, offering hope for a future with fewer HPV-related cancers worldwide.
Cervical Cancer Market
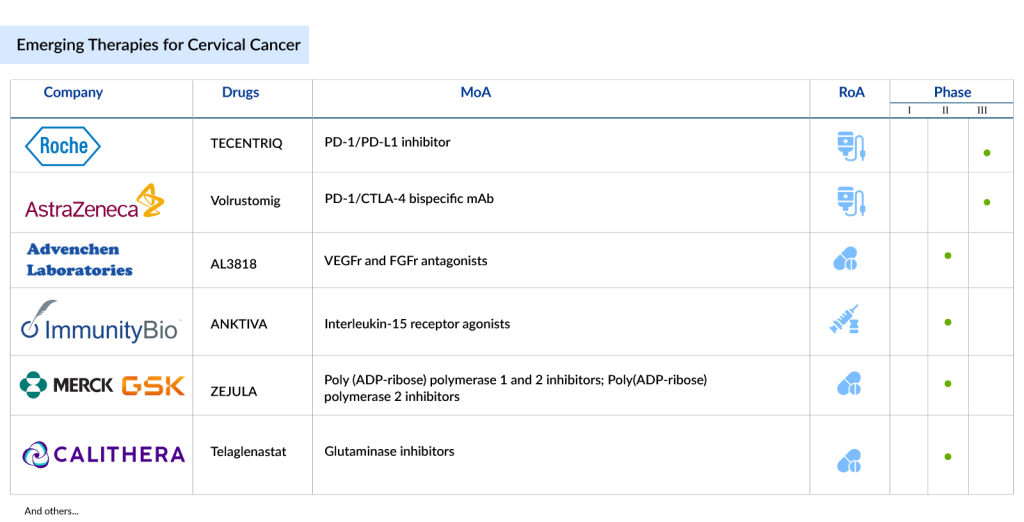
The cervical cancer market is witnessing transformative advancements, particularly in the field of immunotherapy. Leading the charge, Merck’s KEYTRUDA (Pembrolizumab) has already gained approval for PD-L1-positive advanced cervical cancer and is expected to continue its dominance, although it faces emerging competition from Regeneron’s Libtayo (Cemiplimab) and AstraZeneca’s IMFINZI (durvalumab).
Other noteworthy therapies in the pipeline include Iovance Biotherapeutics’ LN-145, a promising tumor-infiltrating lymphocyte (TIL) therapy, and AstraZeneca’s Volrustomig, a bispecific monoclonal antibody targeting PD-1 and CTLA4. Inovio Pharmaceuticals’ VGX-3100, a therapeutic cancer vaccine, also holds promise, despite some challenges in clinical trials. The cervical cancer treatments landscape is expanding rapidly, offering new hope for patients through innovative therapies such as TECENTRIQ (atezolizumab), which has shown significant improvements in survival outcomes when combined with Avastin and chemotherapy.
The cervical cancer market was valued at approximately USD 930 million in 2023 and is expected to grow significantly over the coming years. As ongoing research and clinical trials unlock new treatments, the potential to improve survival rates and quality of life for cervical cancer patients continues to rise.
While challenges like late-stage diagnoses and treatment resistance remain, the evolving treatment options signal a new era in cervical cancer care. The focus on immunotherapies, targeted treatments, and novel vaccines paves the way for more effective, personalized care, ultimately reshaping the global cervical cancer treatment landscape.
Cervical Cancer Pipeline
The cervical cancer treatment pipeline is brimming with innovative therapies that could significantly transform the current landscape. Promising drugs in the pipeline include TECENTRIQ (atezolizumab) by Roche, which, in combination with bevacizumab and chemotherapy, has shown promising results in improving overall survival and progression-free survival in metastatic cervical cancer.
Another notable candidate is Volrustomig by AstraZeneca, a bispecific monoclonal antibody targeting both PD-1 and CTLA4, currently under Phase III trials for high-risk cervical cancer. Other drugs like PRGN-2009 by Precigen, a novel gorilla adenovirus-based therapy, and TG4001 by Transgene, a therapeutic vaccine targeting HPV16, show potential in clinical studies. These treatments, along with others such as ISA101b and ENHERTU, offer hope for more effective treatments, including vaccines and personalized therapies tailored to HPV-driven cancers.
The future of cervical cancer treatment looks increasingly promising, with numerous therapies set to revolutionize care. Drugs like Lifileucel, a one-time personalized T-cell therapy, are expected to be approved by 2025, offering new hope for patients with recurrent or metastatic cervical cancer. As the field advances, a growing focus on biomarker-driven therapies will likely become a central theme, with drugs targeting specific genetic mutations or immune pathways. The market is poised for growth, with new mechanisms of action and the rise of personalized, precision therapies offering substantial improvements in treatment efficacy. As these therapies advance through clinical trials and gain regulatory approvals, they will not only reshape the treatment landscape but also offer patients better outcomes, expanding the range of available options and offering hope for a future where cervical cancer is more effectively managed and treated.
A Future Without Cervical Cancer: Empowering Prevention and Advancing Treatment
Cervical cancer remains one of the most preventable forms of cancer, yet it continues to claim the lives of too many women globally. The introduction of HPV vaccines, advanced screening methods, and groundbreaking therapies has transformed the landscape of prevention and treatment, but the disparity in healthcare access—particularly in low- and middle-income countries—still presents significant challenges. With new immunotherapies, targeted treatments, and promising pipeline drugs like Lifileucel and PRGN-2009, the future of cervical cancer treatment looks brighter than ever. The push for widespread vaccination, early detection, and personalized care is the key to reducing the global burden of cervical cancer. By continuing to innovate, advocate, and ensure equal access to these life-saving measures, we can move closer to a world where no woman loses her life to cervical cancer and prevention becomes the norm, not the exception. The time for action is now—together, we can make cervical cancer a thing of the past.

Downloads
Article in PDF