Chemotherapy-Induced Nausea and Vomiting Side effects
Apr 21, 2017
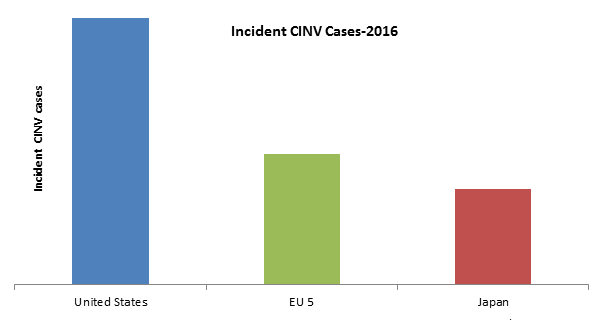
Chemotherapy-induced nausea and vomiting (CINV) are severe side effects of cancer treatment which makes a patient uncomfortable and has to be dealt properly as many patients delay their chemotherapy cycles/ refuse further treatment due to fear of future nausea and vomiting. It is classified as Anticipatory, Acute, Delayed, Breakthrough and Refractory CINV based on the time of CINV occurrence. In comparison to all, Anticipatory CINV is the most difficult one to control. High incidence cases of Chemotherapy-Induced Nausea and Vomiting (CINV) were observed in the United States as compared to EU 5 (United Kingdom, Spain, Germany, France and Italy) and Japan. It is expected that a total of 279,635 Incident CINV cases will be there in the 7 major markets in 2023.
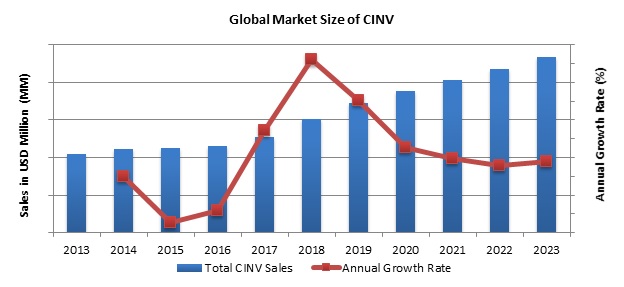
The treatment of CINV is based on the type of CINV occurred/is expected to occur and the chemotherapy regimen patient has undergone. The first line of treatment includes Dexamethasone, Lorazepam, Ondasetron, etc and the second line includes granisetron, domperidone, etc. Usually, a combination of 5-HT3 Antagonist, Dexamethasone, and NK1 receptor antagonist is the most preferred treatment. The Global market for CINV is expected to increase by a CAGR of 8.32%. The drugs- Sancuso, Sustol, Aloxi, Akynzeo and Emend have a strong hold over the CINV market. Apart from these, the expected launch of Pipeline drugs Cinvanti and Rolapitant, the CINV market will be further boosted in the coming years.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Notizia
- Baqsimi wins FDA nod for Hypoglycemia; Freenome captures USD 160M; FDA approval for Ruxience for ...
- CAR-T Cell Therapy aka Miraculous Technology: Mapping the Market, Approved Therapies, Competitive...
- Cancer Cells Trick Body’s Immunity to Survive against Treatment
- Unveiling the Potential of TROP-2 Inhibitors: A New Frontier in Cancer Treatment
With the use of current antiemetic regimens, delayed CINV may be more common than acute CINV as the emetic regimens have more negative effects on quality of life in CINV patients. Although there are a number of drugs in the market for prophylactic treatment of vomiting, there is no drug approved for nausea. There is an unmet need for treatment options when it comes to pediatric CINV patients.
Insight by:
Tejaswini Reddy
Associate Analyst
DelveInsight is a leading Business Consulting and Market Research Firm. We help our clients to find answers relevant to their business, facilitating their decision-making. DelveInsight also serves as a knowledge partner for business strategy and market research. We provide comprehensive analytical reports across various therapeutic indications. DelveInsight has a database of 3000+ high-quality analytical reports.
Downloads
Article in PDF
Recent Articles
- Bright Peak, Ajinomoto to Create Novel Immunocytokines; FDA’s Rejection to Lilly/Pfizer’s T...
- New cancer drug tested in mice may benefit certain leukaemia patients
- Artificial Intelligence and Machine Learning in Software as a Medical Device (SaMD)
- Mitochondria destruction for Cancer Treatment; Atsena raises USD 55 Million financings; Novartis ...
- Generative AI in Drug Discovery: Applications and Market Impact



