Chimeric Antigen Receptor T cell Therapy Market
Jun 30, 2015
Table of Contents
“We designed CAR T cells to overcome a number of problems in getting T cells to attack Cancer”-says Eshhar, after the development of first CAR-T cells (1989).
CAR-T Technologies and History of Innovations
Ever so often in the history of immunotherapy, there comes a breakthrough that takes cancer research across a frontier into a new era. With the development of first CAR-T cells started in the late 1990s and the emergence of new CARs generations with promising study results targeting cancer indications has given a big blow to the immunotherapy market.
Downloads
Article in PDF
Recent Articles
- CAR-T Cell Therapy aka Miraculous Technology: Mapping the Market, Approved Therapies, Competitive...
- Most Common Cancers
- How is Nanomedicine Transforming the Dynamics of the Healthcare Industry?
- AI-Driven Diagnostics: Why are They the Next Big Thing in Healthcare?
- Roche’s Tecentriq combo proves effective in Bladder cancer
Even with these encouraging preliminary findings, the adverse events associated with CAR-T cell therapy are the major drawbacks. To overcome this problem, companies started focusing on the incorporation of safety switches to existing CARs, which are designated to eliminate CAR-T cells in the event of toxicity. The research studies are ongoing with the safety switches, but the success rates are still ambiguous.
Current CAR-T Technology Scenario-Showing promising Results
Researchers are studying ways to improve on the positive results obtained to date by the advancement in the existing CAR technology. First generation CARs having single signaling domain showed very limited clinical benefits. To provide specific targeting and improved function of T-cells, recently, second and third generation CARs have been engineered to include additional stimulatory domains, usually derived from the intracytoplasmatic portion of costimulatory molecules.
Other such technologies are based on safety switches to minimize the adverse events associated with CAR-T cell therapy. Although, some of the companies are conducting their research studies on safety switches with CARs. One of the CAR-T player, Bellicum Pharmaceuticals has developed “safety switch” by the genetic modification of CAR-T cell to kill itself, when exposed to a drug called Rimiducid. The company will start a clinical trial in 2016, incorporating CaspaCIDe safety switch technology with BPX-401, which is a CAR-T product targets CD19 on the surface of blood cancer cells for leukaemia and lymphomas. Other companies like Juno, Intrexon/ Ziopharm and Cellectis are also developing safety switches with their technologies.
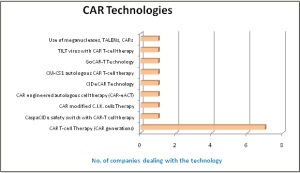
There are 8 different technologies innovated by different leading CAR-T companies, including CIDeCAR and GoCAR-T technology of Bellicum Pharmaceuticals, CAR-eACT technology of Kite Pharma, Use of meganucleases & TALENs by Cellectis and CAR modified C.I.K. cells technology of Formula Pharmaceuticals. More than 50 % of leading firms are using different CAR-T generations.
DelveInsight Expert Reviews
With the entrance of Bio-Pharmaceutical companies, mainly focusing on the advancement of CAR-T technologies are working aggressively to overcome the darkness of cancers. Indeed, more research is needed, that will take nearly 4 to 5 years, before CAR T-cell therapy can be a routine option for patients with cancer. Overall, conditions are currently favorable for the emerging firms to groom their CAR-T business beyond the negatives.
Written by Vikas Soni, Trainee at DelveInsight
For more information about CAR-T cell technology, please read the full report: Chimeric Antigen Receptor (CAR) T cell Immunotherapy- Competitive Landscape, Technology and Pipeline Analysis 2015
For more information on this Report, mail us at info@delveinsight.com
Downloads
Article in PDF
Recent Articles
- Gold Nanoparticles May Soon Treat Prostate Cancer
- Emerging Role of Digital Health in the Field of Oncology
- Juvenescence nets USD 100M; Sarepta DMD drug faces rejection
- Publishers collaborate to remove journal articles from ResearchGate
- Liso-cel Shines in TRANSCEND FL: Impressive Complete Responses, Durable Overall Responses, and Ma...



