Chimeric Antigen Receptor T-cell Therapy Landscape
Jun 17, 2015
Table of Contents
In the field of oncology, no other approach is as attentive as Immunotherapy. Renewed interest in immunotherapy
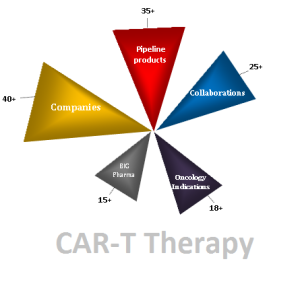
has been driven by Chimeric Antigen Receptor (CAR) T-cell therapy. This emerging approach is (CAR) T-cell technology which is showing great promise in immunotherapy. There are 35+ products in pipeline and 40+ companies are active in this field.
With the advancement of (CAR) T-cell technology showing more safety and efficacy, the companies dealing with immunotherapy are grabbing the opportunity to enter in this new emerging market by the routes of collaborations and acquisitions.
Downloads
Article in PDF
Recent Articles
- Mitochondria destruction for Cancer Treatment; Atsena raises USD 55 Million financings; Novartis ...
- Understanding Rheumatoid Arthritis: Epidemiology Trends and Market Insights
- Gene Therapy Market
- Novartis eyes; Pfizer considers cashing; Mylan pushes to seal $465M; Valeant with Takeda for deal
- Vertex acquires Semma; Luxturna gets approval for rare eye disorder
What is CAR-T Cell Therapy?
CAR T-cells are the engineered T-cells to express chimeric antigen receptor (CARs) which identifies specific antigens of a target-cell. CARs are specifically designed receptors, which graft specificity into an immune effector cell. This therapy acts as genetic modification of T-cells from cancer patients by gene transfer of chimeric antigen receptors (CARs) to recognize molecules on cancer cells without relying on MHC presentation. This adoptive T-cell therapy harnesses the power of the immune system to target and kill tumor cells.
CAR-T Therapy Landscape
Technology
Most of the companies are dealing with the conventional CAR T-cell technology, targeting CD19 antigen of lymphoma cells, which is occupying more than 50% share in target antigens. Meanwhile, the companies are also investing on the studies targeting 25+ different antigens of cancer cells as well as the firms are coming up with 8+ different technologies to enhance the safety and efficacy of the CAR T-cell therapy.
Attractive Pipeline
There are 35+ pipeline products and 40+ companies’ including small and big firms are actively involved. Out of 40 companies, 15+ leading companies are involved with their product in clinical phases. Novartis with the Phase-II product CTL019 is leading the race.
Partnerships
With 25+ collaborations happened in recent years, Juno Therapeutics (JUNO) has inked a massive $727 million R&D deal with Editas Medicine that is considered as greatest deal in this segment. 7+ Research institutes and 12+ small firms having core CAR T-cell technologies partnered with some of the leading companies for the development of CAR-T cell therapy.
Indications
As CAR-T technology are showing most promising results in cancer studies, companies are conducting their Pre-clinical and clinical trial studies with 18+ Oncology indications, in which B-cell malignancies, Lymphoma & Leukemia are the prominent one.
CAR-T Cells Therapy Info-graph
DelveInsight Expert Comments
CAR T-cell therapy will drive the future of Immunotherapy to promising heights. The companies with lagging attention to this approach are already behind. The deals will continue to increase the market size and continued desperation to get on surviving with the key risks on this competitive landscape, numerous players could end up basking in reflected glory.
Written by Vikas Soni, Trainee at DelveInsight
Upcoming DelveInsight Report on CAR-T Cell Therapies-Landscape, 2015
For more information on this Report, mail us at info@delveinsight.com
Let’s share our knowledge, provide your comments in the comment section
Downloads
Article in PDF
Recent Articles
- Emergence of Stem cells and its Market Impact
- Egg Allergy Market: Second most common food allergy but no approved therapy
- Asher Bio raises $55M; Roche halts Huntington’s phase 3 trial; Novartis’ radioligand ...
- Eli Lilly’s Jaypirca Approval; Novartis’ Adakveo EMA Review; Janssen’s CARTITUDE-4 Study of CARVY...
- DelveInsight’s Infectious disorders based Gene Therapy Reports