Nascent Advancements and Emerging Therapies in Chronic Pulmonary Hypertension Treatment Market
Nov 28, 2022
Chronic pulmonary hypertension (CPH) encompasses a heterogeneous group of disorders with the common feature of elevated pulmonary vascular resistance, accounting for approximately 42 million cases in 7MM in 2021. As per DelveInsight’s analysis, the 7MM prevalence is expected to increase at a considerable CAGR of 0.35% during the study period 2019–2032. The CPH prevalence is further divided into four main groups: Group 1, Group 2, Group 3, and Group 4. The most common group of CPH patients in Group 2 CPH (i.e., PH associated with left heart disease). It is estimated that approximately 3-4 million adults in the United States have Group 2 CPH. Furthermore, the World Health Organization (WHO) has organized pulmonary hypertension into five categories depending on the cause. Group 1 primary pulmonary hypertension, or pulmonary arterial hypertension (PAH). Among this group, idiopathic PAH is the most common condition, with other causes such as toxin-induced, connective tissue disorders and other related conditions being less prevalent. Group 2 involves pulmonary hypertension due to left heart disease, which is the most common cause of pulmonary hypertension. Group 3 pulmonary hypertension is due to lung diseases such as COPD and interstitial lung disease. Chronic pulmonary thromboembolism causes group 4 pulmonary hypertension, and group 5 pulmonary hypertension is due to unclear or multifactorial causes.
Chronic pulmonary hypertension (CPH) is a catastrophic disease with high morbidity and mortality and no cure. Despite the rarity of the disease, the potential commercial opportunities for drugs approved for pulmonary hypertension are driven and important by the trend of premium pricing and polypharmacy. Although this chronic pulmonary hypertension market is crowded, commercial interest remains high. More potent and disease-modifying agents fuel this attention, along with the unserviced population lacking approved treatments.
Epoprostenol (Flolan, Veletri) and treprostinil (Tyvaso, Remodulin, Orenitram) can be inhaled, injected, or taken by mouth. The drugs iloprost (Ventavis), bosentan (Tracleer), macitentan (Opsumit), ambrisentan (Letairis), Sildenafil (Revatio, Viagra), and tadalafil (Adcirca, Cialis, Alyq), amlodipine (Norvasc), diltiazem (Cardizem, Tiazac, others), nifedipine (Procardia), Warfarin (Jantoven), Digoxin (Lanoxin), Diuretics, Oxygen therapy, and various other therapies are being used widely to treat the disease.
Downloads
Article in PDF
Recent Articles
- Leman Micro Devices’ e-Checkup system; Calon Cardio-Technology & Leviticus-Cardio announced ...
- ResMed Acquired Somnoware; Stryker Launched Q Guidance System; AlloSource’s AceConnex Pre-Sutured...
- Smith+Nephew’s Hip Arthroplasty System; Mammotome Launched the HydroMARK Plus Breast Biopsy Site ...
- Treatment-resistant Hypertension: Insights into the Challenges and Solutions
- New Clinical Developments in the Pulmonary Arterial Hypertension Treatment Domain
Chronic Pulmonary Hypertension Emerging Therapies
Several key players like Bayer, Attgeno AB, Cereno Scientific AB, Bial – Portela C S.A, Liquidia Technologies, Bellerophon Therapeutics, AbbVie, Tenax Therapeutics, Insmed, Altavant Sciences, and many others are working to bring novel therapies that overcome the roadblocks of existing therapies. Moreover, several potent therapies like Supernitro, CS1, Yutrepia, Zamicastat, and many others existing in different stages can potentially be better rivals for therapies existing in the PAH market.

Attgeno AB’s Supernitro (Phase-II) is the first and only intravenous drug to treat acute high blood pressure in the lungs selectively. Supernitro working on Attgeno technology is based on ground-breaking research on nitric oxide and circulatory physiology. The method releases nitric oxide to specific organs in the body. It generates a range of new drug substances with unique properties that can be used in life-threatening medical conditions. Supernitro represents a great new step in the medical use of nitric oxide. The drug is developed to widen the blood vessels in the lungs selectively.
Cereno Scientific AB’s drug candidate CS1 (Phase-II) is a new advanced reformulation of valproic acid (VPA) and acts as an epigenetic modulator with anti-thrombotic, anti-inflammatory, anti-fibrotic, and pressure-relieving properties. CS1 is being developed as pulmonary arterial hypertension (PAH) treatment to offer patients a better, disease-modifying drug. CS1’s epigenetic mechanism is expressed through HDAC (histone deacetylase) inhibition. It brings a novel treatment approach to cardiovascular disease.
Liquidia Technologies’s Yutrepia (LIQ861) is an inhaled dry powder formulation of treprostinil designed using Liquidia’s PRINT technology to enhance deep-lung delivery using a convenient, palm-sized, disposable dry powder inhaler (“DPI”) for the treatment of PAH is currently registered. Liquidia believes LIQ861 can overcome the limitations of current inhaled therapies and has the potential to maximize the therapeutic benefits of treprostinil in treating PAH by safely delivering higher doses into the lungs.
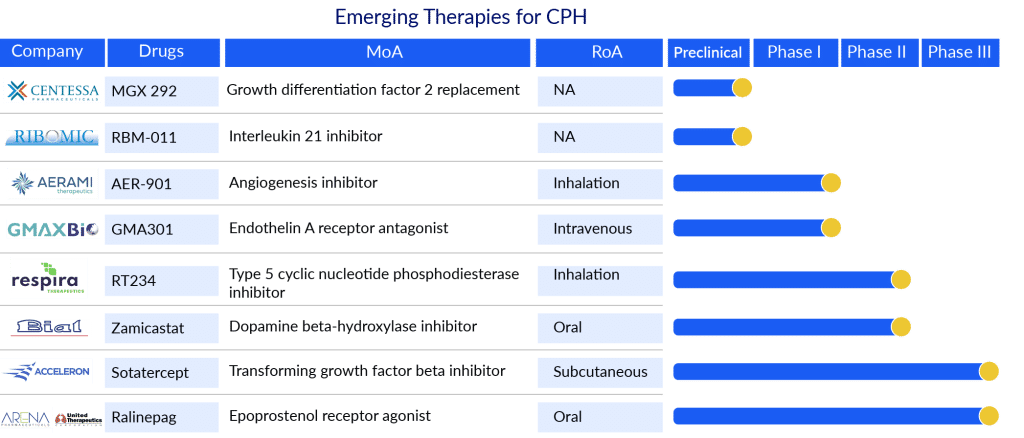
Recent years have witnessed considerable progress in Chronic PH. Well-established pathways and existing therapies are being refined and extended by introducing novel compounds. Furthermore, Bial – Portela C S.A.’s Zamicastat (BIA 5-1058), Bellerophon Therapeutics’ INOpulse® A, and AbbVie/Tenax Therapeutics’ Levosimendan are currently present under different phases and are expected to show promising results.
Recent Developments in the Chronic Pulmonary Hypertension Treatment Market
- In July 2022, Cereno Scientific announced that the first patient had been enrolled in the Phase II study in pulmonary arterial hypertension (PAH) with drug candidate CS1. Based on the timing of enrollment and several factors mainly related to the activation of clinical sites, the study timeline has been adjusted by about a quarter, and top-line results are now estimated for Q1 2023. The number of study sites has increased to include about ten clinics across the US, with the potential for further expansion to meet the Q1 timeline.
- In June 2022, Gossamer Bio, Inc. announced the publication of key preclinical data supporting the potential of seralutinib for treating pulmonary arterial hypertension (PAH). Inhaled seralutinib was an effective treatment of severe PAH in two animal models, with improved cardiopulmonary hemodynamics, reduction in NT-proBNP, reverse remodeling of pulmonary vascular pathology, and improvement in inflammatory biomarkers. Seralutinib showed greater efficacy compared to imatinib in a preclinical study.
- In June 2022, Keros Therapeutics, Inc. announced results from a preclinical study of RKER-012 on cardiac and pulmonary pathology in an established rodent model of pulmonary arterial hypertension (“PAH”), which were presented at the Pulmonary Hypertension Association International Conference and Scientific Sessions held on June 10 through 12, 2022.
- In June 2022, Attgeno AB announced that it had received approval from the Swedish Medical Products Agency and the Swedish Ethical Review Authority to start a phase 2 clinical trial of its lead drug candidate Supernitro as a potentially lifesaving treatment for acute pulmonary hypertension patients after cardiac surgery.
- In May 2022, Anumana, Inc., an AI-driven health technology company from nference, Inc., developed in collaboration with Janssen and Mayo Clinic, was granted Breakthrough Device Designation for its AI-enhanced, ECG-based Pulmonary Hypertension (PH) Early Detection Algorithm by U.S. Food & Drug Administration (FDA).
- In May 2022, Aerovate Therapeutics, Inc. presented Phase 1 results at the American Thoracic Society (ATS) International Conference in San Francisco. Aerovate’s data showed that AV-101, a novel inhaled dry powder formulation of imatinib, was generally well-tolerated by healthy adult volunteers with no serious adverse events reported. AV-101 is being developed to address cellular hyperproliferation and resistance to apoptosis in the pulmonary vasculature, which are key features of the pathophysiology of PAH.
- In May 2022, Altavant Sciences presented results from a preclinical study comparing rodatristat ethyl as a monotherapy and in combination with the type A endothelin receptor antagonist, ambrisentan, in an animal model of pulmonary arterial hypertension (PAH). Results describing changes in post-hypoxia occlusions and mean pulmonary arterial pressure (mPAP) with each treatment regimen were presented in a poster at ATS 2022 held in San Francisco, CA, May 13-18, 2022.
- In April 2022, Respira Therapeutics announced that the first patient in the United States had been dosed in the multicenter trial of its lead product candidate, RT234-PAH (vardenafil administered as a dry powder inhaled treatment). The VIPAH-PRN 2b trial (Vardenafil Inhaled for Pulmonary Arterial Hypertension – PRN) will consist of two sequential cohorts receiving RT234 as single doses administered via an Axial Oscillating Sphere dry powder inhaler. The study is designed to evaluate the safety and preliminary efficacy of RT234 to acutely improve episodic symptoms and exercise capacity in people being treated for pulmonary arterial hypertension (PAH) with New York Heart Association (NYHA) Functional Class II-III symptoms.
- In March 2022, Resverlogix Corp. announced the publication of an article entitled “BET Protein Inhibition for Pulmonary Arterial Hypertension: A Pilot Clinical Study” in the prestigious American Journal of Respiratory and Critical Care Medicine. The article outlines the positive impact of apabetalone in the investigator-led pulmonary arterial hypertension (PAH) pilot study, APPRoAcH-p.
- In July 2021, PulmoSIM Therapeutics announced that it had entered into a strategic partnership with investigators from National Jewish Health and Brown University for the clinical development of PT001. This drug targets multiple responsible pathways in PAH to provide curative treatment. The Food and Drug Administration (FDA) granted orphan drug designation for PT001 for treating PAH.
The Changing Paradigm of the Chronic Pulmonary Hypertension Market
Pulmonary Hypertension is a constantly evolving domain in which clinicians encounter a plethora of diagnostic and treatment issues. There is significant variety in Pulmonary Hypertension, which seldom manifests as an independent entity and is more frequently coupled with one or more predisposing factors, exposures, or disease states. The rates of clinical disease development and results vary greatly amongst people, and there is no generally successful Chronic Pulmonary Hypertension treatment method, even within a particular form of Pulmonary Hypertension. Because of these difficulties, continuous efforts to develop and standardize disease diagnosis, categorization, and management techniques are critical. These factors are expected to enhance the Chronic Pulmonary Hypertension market growth worldwide.
Moreover, promising pipelines and successful drug approvals have given the Chronic Pulmonary Hypertension market a considerable boost in recent years. The extensive research and development activities of massive pharma key players and the launch of various multiple-stage pipeline therapies are expected to dominate in the near future. The number of clinical trials for Chronic Pulmonary hypertension (CPH) is also increasing; therefore, their success rate is also boosting the chances of commercial approval during the forecast period 2019–2032. This is also a major factor in the growth and development of the Chronic Pulmonary Hypertension market. Furthermore, the government is also taking active steps that promote the growth of the Chronic Pulmonary Hypertension market. In addition, the increasing prevalence and awareness of the disease also drive the Chronic Pulmonary Hypertension market. Hence due to the aforementioned factors, the treatment market is expected to grow in the upcoming years.

FAQ
Pulmonary hypertension encompasses a heterogeneous group of disorders with the common feature of elevated pulmonary vascular resistance. Pulmonary hypertension is a progressive disease with treatment focused on managing symptoms and treating underlying diseases.
Some common underlying causes of pulmonary hypertension include high blood pressure in the lungs’ arteries due to some types of congenital heart disease, connective tissue disease, coronary artery disease, high blood pressure, liver disease (cirrhosis), blood clots to the lungs, and chronic lung diseases like emphysema. Genetics also plays a role.
The symptoms of pulmonary hypertension during the initial stage of the disease are common to many other medical conditions (e.g., difficulty breathing and fatigue). This often results in a delayed diagnosis until more severe symptoms arise, such as dizziness, chest pain, ankle swelling, or feeling the heart race or pound (palpitations).
Diagnosis of pulmonary hypertension is commonly delayed as the presenting symptoms overlap with other disease processes. Other conditions to be considered in the differential diagnosis include, but are not limited to, congestive heart failure, coronary artery disease, pulmonary fibrosis, chronic obstructive pulmonary disease, valvular heart disease, hypothyroidism, and pulmonary embolism.
Downloads
Article in PDF
Recent Articles
- One of the leading causes of blindness, and a sight-threatening disease, Uveitis, now has novel t...
- ResMed Acquired Somnoware; Stryker Launched Q Guidance System; AlloSource’s AceConnex Pre-Sutured...
- Ethicon’s ETHIZIA Hemostatic Sealing Patch; FDA Approves Medtronic’s Minimally Invasive Device to...
- World Hypertension day
- Smith+Nephew’s Hip Arthroplasty System; Mammotome Launched the HydroMARK Plus Breast Biopsy Site ...