Towards a Promising Future: Unveiling Advancements in Chronic Spontaneous Urticaria (CSU) Treatment
Aug 14, 2023
Table of Contents
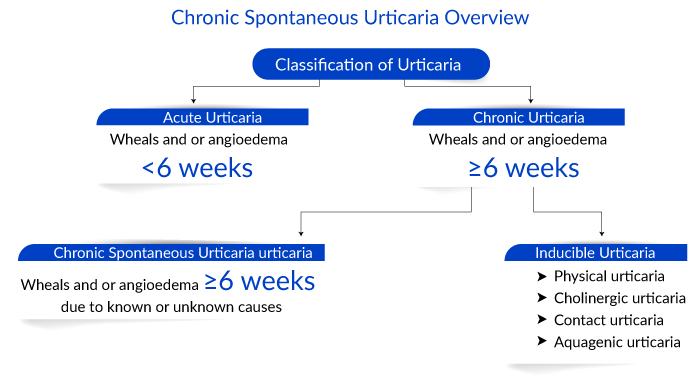
Chronic urticarial is a common skin condition characterized by itchy, wheal-and-flare skin reactions or hives. As per Delveinsight analysts, in 2022, there were around 776K individuals in the US affected by chronic urticaria. It can be spontaneous or inducible, lasting more than 6 weeks and persisting for over a year. In contrast to chronic spontaneous urticaria (CSU), where the cause is unknown, chronic inducible urticaria (CIndU) has definite and specific triggers that induce symptoms.
What is chronic spontaneous urticaria?
Chronic spontaneous urticaria, also known as chronic idiopathic urticaria, is a debilitating disease. It results from the pathogenic activation of mast cells and basophils that release pro-inflammatory mediators that support the generation of urticaria. The major characteristics of CSU are itching and hives with or without angioedema lasting more than 6 weeks. The hallmark symptom is the presence of red, swollen, itchy, and sometimes painful hives or “wheals” on the skin. These lesions can be as small as a few millimeters in diameter but can coalesce to form ‘wheals’ as large as several centimeters wide. Patients are highly affected by the disease and direct and indirect healthcare costs can become significant with 20–30% diminished job performance among patients.
Downloads
Article in PDF
Recent Articles
- Neurotech’s ENCELTO Becomes First FDA-Approved Treatment for MacTel Type 2; Plus Therapeutics’ Rh...
- Sanifit nets USD 80.9 M; FDA approves Dupixent for CRS; AbbVie buys Allergan
- The Evolving Landscape of COPD Treatments: New Hope for Patients
- FDA Approves Sanofi/Regeneron’s DUPIXENT as First New CSU Therapy in Over a Decade; Gilead’s TROD...
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
Diagnosing the itch: Chronic spontaneous urticaria
Women are twice as likely as men to be diagnosed with the disease, and most people develop their first chronic spontaneous urticaria symptoms between 20 and 40 years of age. Diagnosing chronic spontaneous urticaria involves a thorough physical examination and medical history. However, additional tests such as blood tests, allergy tests, or skin biopsies are occasionally performed to rule out other possible causes of urticaria, like allergic reactions or infections. Further, tools like urticaria activity score (UAS), urticaria control test (UCT), chronic urticaria quality of life, urticaria severity score (USS), and others are used to monitor and evaluate disease activities.

An autologous serum skin test is another diagnostic procedure used to diagnose CSU due to auto-reactivity. Due to auto-reactivity, chronic spontaneous urticaria is defined by the presence of circulating mast cell activating signals. In some patients, these mast cell activators appear to be autoantibodies directed against the high-affinity IgE receptor (FcεRI) or against IgE itself.
Current approaches to chronic spontaneous urticaria treatment
Chronic spontaneous urticaria treatment is challenging; the current treatment comprises a regime to alleviate symptoms and prevent a recurrence. The chronic spontaneous urticaria treatment pattern typically involves a step-wise approach, starting with first-line treatments such as antihistamines and corticosteroids and progressing to more advanced options if necessary. Further, chronic spontaneous urticaria treatment patterns vary depending on the individual and their response to medications. Various factors are to be considered to create the most appropriate treatment plan, such as the severity of symptoms, chronic spontaneous urticaria treatment response, and any underlying conditions.The international APAAACI/EAACI/GA²LEN/EuroGuiDerm guidelines recommend second-generation H1-antihistamine as first-line treatment for all types of urticaria. They work by blocking histamine H1 receptors in the body, alleviating the chronic spontaneous urticaria symptoms of itching, redness, and swelling associated with CSU.
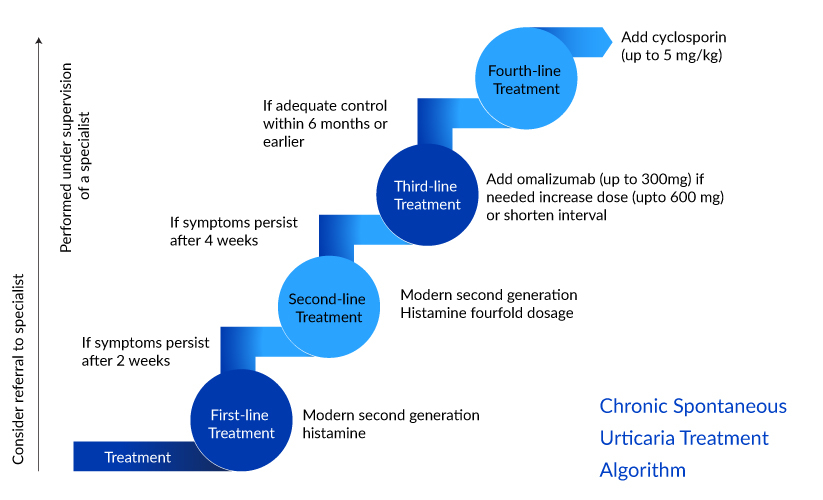
The second-line chronic spontaneous urticaria treatment recommended is up-dosing of second-generation H1– antihistamine up to fourfold in patients with chronic urticaria unresponsive to the standard dose, before other treatments are considered. XOLAIR (omalizumab), a monoclonal antibody, is the only licensed therapy approved and the guidelines recommend it as a third-line treatment for treating patients with chronic urticaria who are unresponsive to high-dose antihistamines. However, almost one-third of patients remain symptomatic despite the use of omalizumab.
Cyclosporine is recommended only in patients with severe disease, refractory to any dose of antihistamine and omalizumab in combination. Other symptomatic off-label chronic spontaneous urticaria therapies like corticosteroids, antidepressants, immunosuppressants, leukotriene receptor antagonists, sulfasalazine, methotrexate, interferon, plasmapheresis, phototherapy, IV immunoglobulins are recommended options based on individual cases.
The American Academy of Allergy, Asthma, and Immunology (AAAAI) represents a divergence from Europe and other guidelines worldwide, by keeping first-generation H1-antihistamines in the treatment algorithm. Most countries recommend avoiding first-generation H1-antihistamines altogether.
Advancements in the field of chronic spontaneous urticaria treatment space
Decades of research and development have led to significant advances in chronic spontaneous urticaria with several revisions in the classification of urticaria, clinical guidelines, management, and diagnosis.
- Improved disease understanding: Advancements in research have shed more light on the complex mechanisms underlying chronic spontaneous urticaria. Several hypotheses have been proposed to pinpoint the disease’s cause. The presence of an autoimmune angle, abnormalities of tissue mast cells and basophils, as well as other serologic markers, and the participation of blood coagulation factors, toll-like receptors, and IgG anti-FcRI alpha, are identified. Although the etiology of CSU is still unknown, knowledge has improved.
- Updated terminology/classification: The older terminology of chronic autoimmune urticaria or chronic idiopathic urticaria is now differentiated from physically induced urticaria. Further, physical urticaria has been revised to chronic inducible urticaria (CIndU) to reflect the external trigger and inducible nature. CIndU also includes cholinergic, aquagenic, and contact urticaria; however, the remaining forms of urticaria, which occur without an external trigger, are called CSU or chronic idiopathic urticaria.
- Development of tools: Newer prospective and retrospective tools have emerged that quantify the effect of urticaria on quality of life. Urticaria activity score (USA7) is a prospective tool that records the severity of itching and the number of wheals daily for 7 days. Urticaria control test, chronic urticaria quality of life questionnaire, and urticaria severity scores are examples of retrospective tools that have been developed.
- Upgrades in clinical guidelines: Multiple guidelines have been highlighted, although the EAACI/GA2LEN/EDF/WAO remains the most popular among practicing clinicians. The newest guidelines possess a step-wise approach and simpler treatment algorithm for treating CSU. Further, the guidelines have included omalizumab as a third line of treatment after the four times dose-escalation of second-generation H1 antihistamine.
The advancement in clinical guidelines with GRADE methodologies has offered evidence-based diagnostic and therapeutic approaches toward different subtypes of urticaria. Tools to assess the impact of chronic urticaria on quality of life track and monitor the disease progression and management. However, there is a considerable need for progress in the identification of biomarkers to clarify disease pathogenesis and etiology.
Unfolding emerging refractory chronic spontaneous urticaria treatment options
Despite a well-established ladder approach to treatment, there is a paucity of effective options, and almost one-third of patients remain symptomatic after omalizumab. Treatments with H1-antihistamines and omalizumab have so far not always been successful in adequately controlling intense pain and itching. The development of therapeutic modalities targeting novel pathways will hopefully address these unmet needs for chronic spontaneous urticaria patients. Some of these novel molecules include BTK inhibitors, interleukin inhibitors, siglec-8-directed agonist, TSLP inhibitors, KIT inhibitors among others.
Novartis’ Remibrutinib (LOU064) is an oral BTK inhibitor with remarkable kinase selectivity and FcR1-mediated activity. With rapid onset of action and a favorable safety profile, it can become the first in class to fulfill the need for rescue medication, achieve complete symptom control without any itch or pain, and normalize quality of life (QoL) for chronic spontaneous urticaria patients.
For patients with insufficient response to omalizumab, numerous other biologicals are currently under development such as dupilumab, tezepelumab, lirentelimab, benralizumab, barzolvolimab, and others.
Sanofi and Regeneron’s DUPIXENT (dupilumab) inhibits IL-4 signaling via the ‘Type I’ receptor and both IL-4 and IL-13 signaling through the ‘Type II receptor. Multiple cell types that express IL-4Rα (e.g., mast cells, eosinophils, macrophages, lymphocytes, epithelial cells, goblet cells) and inflammatory mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines, chemokines, nitric oxide, and IgE) are involved in inflammation. Many of these cells have been linked in CSU, and inhibiting them all at once could impart very effective control to CSU compared to XOLAIR.
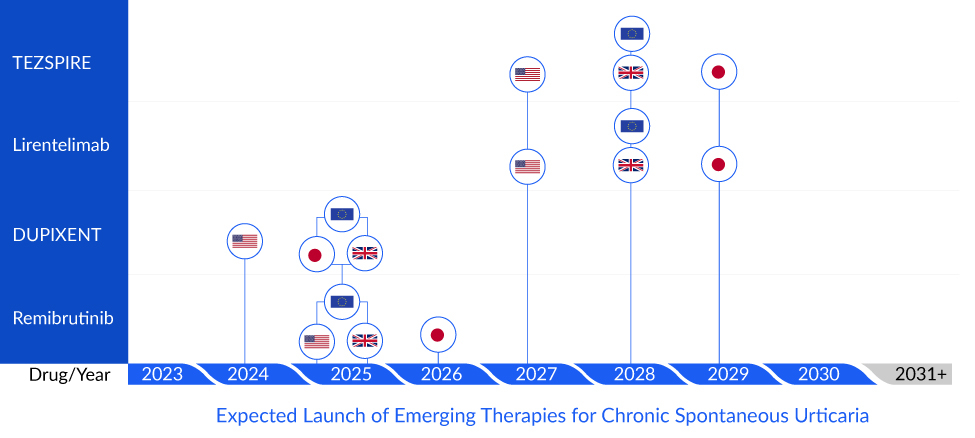
TEZSPIRE (tezepelumab) being developed by AstraZeneca and Amgen is a prefilled single-use auto-injector that blocks thymic stromal lymphopoietin that can potentially prevent and treat the lesional skin. Further, it will provide patients the ease of administration, as the subcutaneous administration of XOLAIR burdens the healthcare infrastructure and patients.
Allakos and BioWa’s lirentelimab is a Siglec-8 agonist which is an inhibitory receptor in eosinophils and mast cells. Further, lirentelimab’s engineered potent antibody-dependent cellular cytotoxicity provides an additional potent way to deplete eosinophils. The drug with its dual mechanism of action has the potential to treat omalizumab naïve and failed patients.
The upcoming chronic spontaneous urticaria drugs will potentially diminish XOLAIR’S stronghold in the market. However, they further require safety and efficacy data to validate their potential and support regulatory approval. Most emerging therapies are an add-on to current therapies and are being developed for refractory cases. Therefore, there exists the need to develop new drugs as first-line curative therapies.
Although there has been tremendous investment in CSU, there are still gray areas that require attention. Epidemiology studies involving children and adolescents are limited, and most studies do not distinguish between relevant chronic urticaria subtypes. Further, the underlying cause is still unknown, forming a hindrance to the development of effective and curative approaches. According to DelveInsight’s estimates, the total chronic spontaneous urticaria market size of the seven major markets (the United States, Germany, France, Italy, Spain, the United Kingdom, and Japan) is likely to cross the USD 6,000 million mark by 2032 owing to the launch of biologics, increased awareness, and improved diagnosis.
FAQs
Chronic urticaria is spontaneous or inducible, lasts >6 weeks, and persists for >1 year. It impacts the quality of life and is linked to psychiatric comorbidities and high healthcare costs, often causing huge socio-economic distress for the patients.
The etiology of chronic spontaneous urticaria is yet to be fully established. The exact cause is often unknown, but it may be due to autoimmune reactions wherein the immune system mistakenly targets healthy cells in the skin. Other potential triggers include medications, infections, insect bites, stress, and temperature changes.
The diagnosis is based on a physical examination and medical history. Additional tests are performed to rule out underlying causes or to identify triggers, such as blood tests, allergy tests, or skin biopsies. Screening tests for thyroid function and antithyroid peroxidase and antithyroglobulin antibodies are recommended. Positive autologous serum skin test (ASST) and in vitro testing of the patient’s serum for the anti-FCeRIa or the anti-IgE autoantibodies by basophil histamine release assay (BHRA) is also recommended.
Treating chronic spontaneous urticaria is challenging, and the therapeutic goal is a reduction in disease activity, complete symptom control, and improvement in QoL. The current treatment regime aims to alleviate symptoms and prevent their recurrence. The treatment pattern typically involves a stepwise approach, starting with first-line treatments and progressing to more advanced options.

Downloads
Article in PDF
Recent Articles
- The Evolving Landscape of COPD Treatments: New Hope for Patients
- ATS 2023 Updates: Dupixent – A Ray of Hope For Moderate To Severe Chronic Obstructive Pulmo...
- Major Highlights and Insights from the AAAAI Annual Meeting, 2024
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
- Can Dupixent Be A Gamechanger In The Atopic Dermatitis Treatment Landscape?