DARZALEX FASPRO Regimen Approval: Johnson & Johnson Howling Success in Multiple Myeloma
Aug 05, 2024
Despite significant progress in multiple myeloma treatment with a range of biological drugs and their combinations—such as IMiDs (BMS REVLIMID, POMALYST/IMNOVID), anti-CD38 monoclonal antibody drugs (DARZALEX, SARCLISA), the anti-SLAM7 monoclonal antibody (AbbVie and BMS multiple myeloma drug EMPLICITI), and new proteasome inhibitors (KYPROLIS, NINLARO)—relapse after initial therapy remains a common issue for many patients.
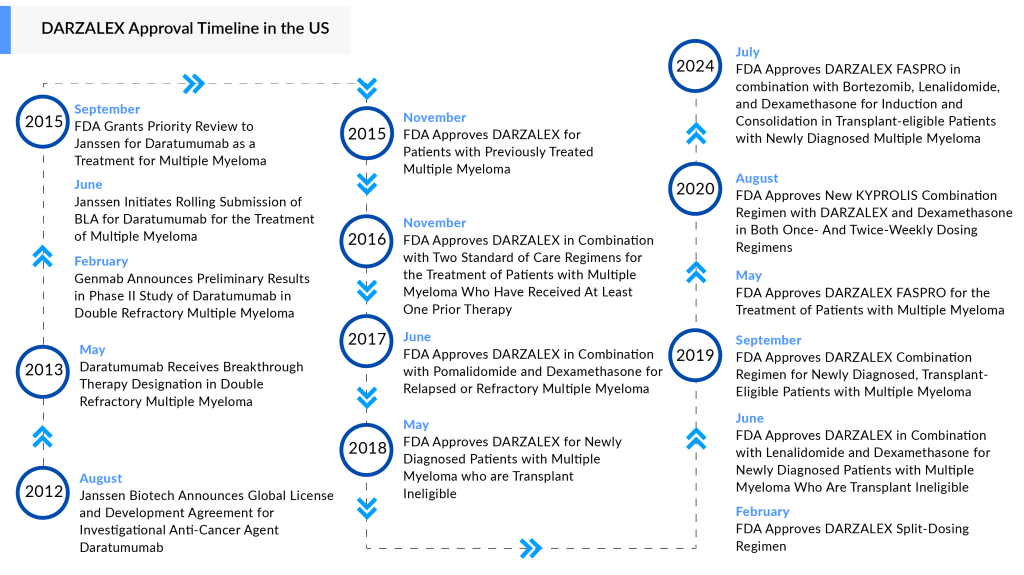
In frontline clinical trials for multiple myeloma, Janssen multiple myeloma drug DARZALEX has proven to be effective and is now considered the standard of care. It has surpassed expectations in terms of both efficacy and safety and is anticipated to dominate the multiple myeloma market.
A second CD38 multiple myeloma drug, SARCLISA, has also been approved for multiple myeloma and is rapidly gaining traction in key markets. However, DARZALEX has a lead of over four years in the field. Both anti-CD38 monoclonal antibody drugs are competing in quadruplet regimens for both transplant-eligible and ineligible patients, and are nearing a crucial comparison in non-transplant-eligible patients. Recent evidence for CD38-RVd combinations is emerging from studies involving transplant-eligible patients.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Bristol Mayer Squibb — The Undisputed Leader in the Molecular Glue Arena
- Rising of Orphan Drug Development
- Kiadis inks license deal with Sanofi; Gilead Ends Hep B Collaboration; Merck & Foghorn inks ...
- The Promise of NK Cells: A Novel Approach to Immunotherapy
- CEPI Grants $41.3 Million to Valneva; Innovent Achieves Phase III Success for Mazdutide; GSK’s BL...
DelveInsight’s data suggests that roughly half of newly diagnosed multiple myeloma patients are ineligible for transplant, and around a third of eligible patients do not receive the transplant. In the US, there were around 23,600 frontline transplant-ineligible patients and 9,200 transplant-eligible patients of multiple myeloma patients in 2023.
Following the groundbreaking trial results of the DARZALEX FASPRO regimen shared at last year’s American Society of Hematology meeting, Johnson & Johnson is launching what it refers to as a “cornerstone frontline therapy” for multiple myeloma, while also broadening its significant footprint in the field.
On 30 July 2024, the FDA authorized the use of DARZALEX FASPRO in combination with VRd—a regimen that includes Takeda’s VELCADE, BMS’s REVLIMID, and the steroid dexamethasone—for induction and consolidation therapy in patients with newly diagnosed multiple myeloma who are candidates for an autologous stem cell transplant.
DARZALEX FASPRO was granted FDA approval in May 2020 and is approved for nine different uses in multiple myeloma. Four of these indications are for frontline multiple myeloma treatment in newly diagnosed patients, whether they are eligible or not for a transplant.
DARZALEX FASPRO is the only subcutaneous CD38-directed antibody approved for treating multiple myeloma patients. DARZALEX FASPRO is combined with recombinant human hyaluronidase PH20 (rHuPH20), utilizing Halozyme’s ENHANZE drug delivery technology. In August 2012, Janssen Biotech, Inc. and Genmab A/S entered into a global agreement, giving Janssen an exclusive license to develop, produce, and market daratumumab.
The franchise earned $9.74 billion globally last year. In addition to the DARZALEX franchise, other Janssen multiple myeloma treatment drugs include the CAR-T therapy CARVYKTI, developed in partnership with Legend Biotech, as well as the multiple myeloma bispecific drugs TECVAYLI and TALVEY.
This new multiple myeloma drug approval is based on data from the Phase III PERSEUS study, which assessed DARZALEX FASPRO in a treatment regimen involving D-VRd induction and consolidation therapy, compared to bortezomib, lenalidomide, and dexamethasone (VRd) during the same phases in patients with newly diagnosed multiple myeloma (NDMM) who were eligible for autologous stem cell transplant (ASCT). After consolidation, patients were given an investigational maintenance regimen that included DARZALEX FASPRO with either lenalidomide or lenalidomide alone.
“Multiple myeloma presents a diverse clinical progression in patients and even within individuals, highlighting the ongoing need for innovative therapies that target various factors and combinations. This is crucial for offering effective treatment options both at diagnosis and throughout the disease’s progression,” stated Amrita Y. Krishnan, M.D., Professor and Director of the Judy and Bernard Briskin Multiple Myeloma Center at City of Hope. “The efficacy data supporting this new quadruplet regimen, along with its proven safety and tolerability, strongly suggest that incorporating D-VRd at the initial diagnosis, compared to VRd, can enhance responses and extend remission durations in the context of autologous stem cell transplantation.”
The PERSEUS study findings revealed a notable enhancement in the primary endpoint of progression-free survival (PFS), with D-VRd reducing the risk of disease progression or death by 60% compared to VRd (HR [95% CI]: 0.40 [0.29, 0.57]; p-value < 0.0001). The use of D-VRd for induction and consolidation led to deeper responses at the end of consolidation relative to VRd, with minimal residual disease (MRD) negativity rates of 57.5% versus 32.5%, and MRD-negativity rates among patients with complete response (CR) or better of 76.6% versus 58.5%, respectively.
Jordan Schecter, M.D., Vice President and Disease Area Leader for Multiple Myeloma at Johnson & Johnson, stated, “The recent approval of DARZALEX FASPRO-based quadruplet therapy shows a notable decrease in disease progression or death during first-line treatment, especially when patients are expected to achieve their most significant responses. This approval reflects our dedication to establishing new benchmarks in care for patients newly diagnosed with multiple myeloma who are eligible for a transplant.”
The safety profile of D-VRd was in line with the established safety profiles for DARZALEX FASPRO and VRd. The most frequently reported side effects (≥20%) in patients with multiple myeloma receiving D-VRd include peripheral neuropathy, fatigue, edema, fever, upper respiratory infections, constipation, diarrhea, musculoskeletal pain, insomnia, and rash.
This approval has put Janssen’s multiple myeloma drug DARZALEX in the driver’s seat. However, it will get stiff competition from the other pharma companies who are trying their best to grab some market share in this competitive space.
Several key players are racing to bring their candidates to the multiple myeloma treatment market, and we estimate that many bispecific antibodies and CAR-Ts will join the fray by 2025. Some of the frontrunners in this space are Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, Sanofi, Bluebird bio, Takeda, Amgen, Oncopeptides AB, AbbVie, Roche (Genentech), Karyopharm, Arcellx, Novartis, TeneoOne, CARsgen Therapeutics, Regeneron Pharmaceuticals, and others.
It will be interesting to see how DARZALEX maintains its position against these upcoming multiple myeloma therapies.

Downloads
Article in PDF
Recent Articles
- FDA Approves BMS’s Reblozyl for MDS; FDA Awards Orphan Drug Designation to NXC-201; Janssen Submi...
- EC grants; Pfizer cuts; 27 medicines sold; Keytruda nabs
- BeiGene’s BRUKINSA Gets FDA Accelerated Approval; GSK’s Positive Results in DREAMM-8 ...
- Leo Pharma’s Hand Eczema Clinical Trial Updates; GSK’s PD-1 inhibitor Jemperli Approval; Ro...
- AZ offloads; Pfizer’s deal; Takeda’s work on plant; Germany’s doc payment



