Treating Patients with Software: Digital Therapeutics
Dec 11, 2020
The 3rd generation pharmaceuticals- Digital therapeutics- have become a much-discussed aspect of MedTech today, as they compete with established drug-based pharmaceutical and biotech products to become therapeutic interventions for many acute and chronic diseases. Currently, many small- and mid-size companies are working to develop, manufacture and launch their software-based therapeutic interventions, while we are observing a significant growth of acquisitions, licensing deals, and collaborations among the big-pharma players, all vying to have a niche in this arena.
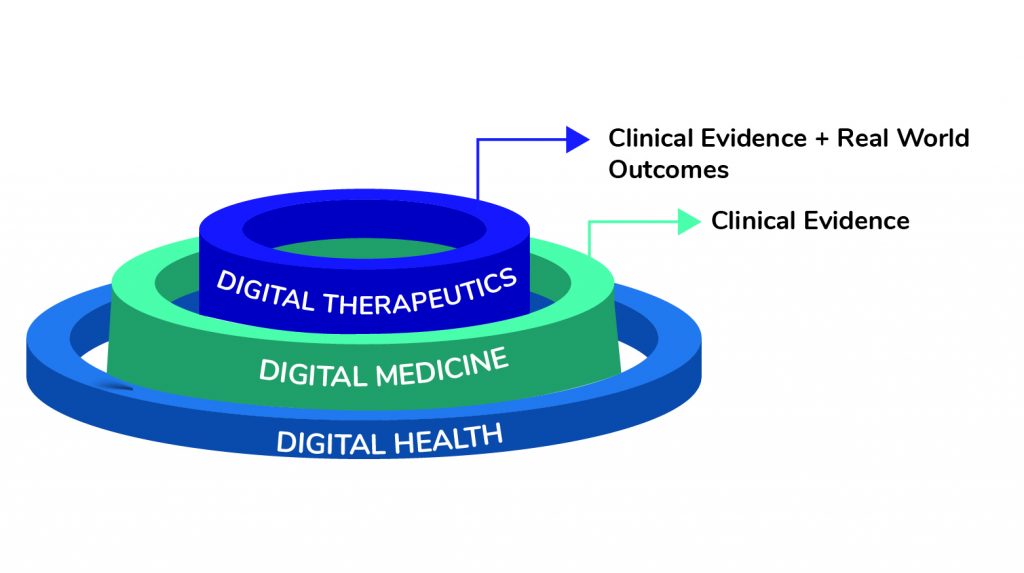
As the digital aspect merges across the healthcare ecosystem, digital therapeutics are expected to influence healthcare delivery across the world. Unlike traditional prescription drugs rather than swallowing a pill or taking an injection, patients are treated with software.
Recent years witnessed Digital therapeutics as an emerging alternative to traditional medicine for mental health and addiction treatment. According to the WHO, more than 250 million people suffer from depression and mental health-related problems every day and it is the leading cause of disability and suicides. Although there are reasonably effective treatments for depression available such as the antidepressants, there aren’t enough healthcare resources to treat the growing number of people with depression.
Downloads
Article in PDF
Recent Articles
- Johnson & Johnson’s Tecnis PureSee lens; Sparrow BioAcoustics’s Stethoscope Software; Better ...
- We’re all in this together!
- Potential of Digital therapeutics and increasing CVDs
- Plotting the Extensive Demand of Mobile Apps for Mental Health
- Watch Out For Top Pipeline Therapies Making An Impact In The Bipolar Depression Market
Digital therapeutics utilize digital solutions to change patient behavior and lifestyle, usually with the help of a smartphone and are delivered through an app. Some digital therapeutic products have been successfully used to treat many chronic diseases like type II diabetes, obesity, and depression. With the help of digital therapeutics, healthcare professionals can easily connect with the patient on an online platform and enable personalized and real-time treatment methods.
There are several companies involved in digital therapeutic development, one notable of them being Pear Therapeutics. Pear’s lead product, reSET, is a promising candidate for the treatment of Substance Use Disorder, and was the first DTx to receive authorization from FDA to improve disease outcomes. Pear’s other product, reSET-O for the treatment of Opioid Use Disorder, was the first to receive Breakthrough Designation and was authorized in December 2018. Being FDA approved, these tools are prescribed as cognitive behavioral therapy as an adjunct to outpatient treatment under the supervision of a clinician. Reset-O is also intended to be used in conjunction with pharmacotherapy. Pear’s third product, Somryst for chronic insomnia treatment, was the first product submitted through FDA’s traditional 510(k) pathway while simultaneously reviewed through FDA’s Software Precertification Pilot Program and was authorized in March 2020.
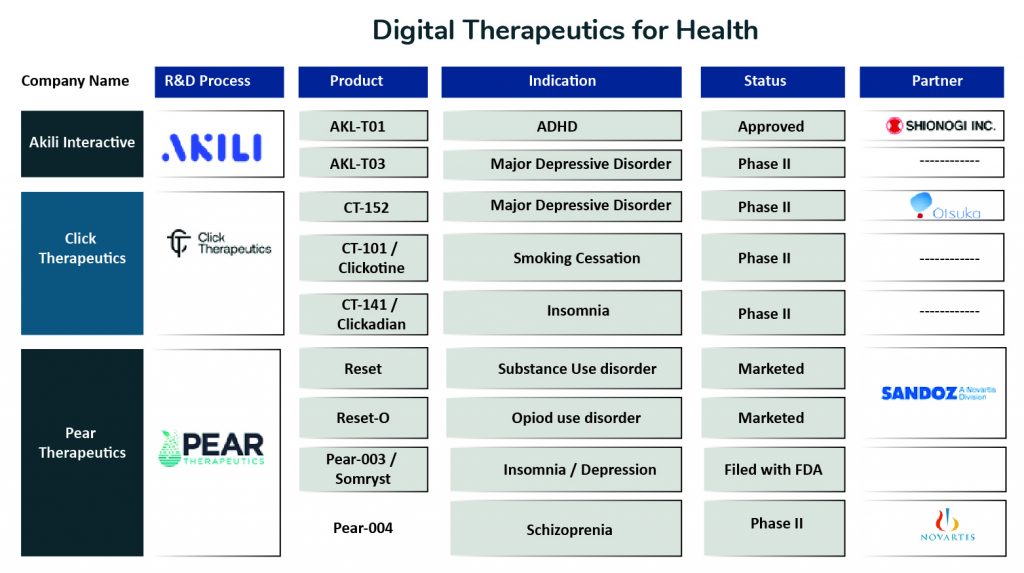
Another company active in this space is Akili Therapeutics, which is working to create a niche for themselves by applying gaming principles in healthcare to improve patient clinical outcomes. In June 2020, the FDA permitted the marketing of Akili Interactive Labs, Inc. product EndeavorRx, the first Game-based Digital Therapeutics to improve attention function in children with ADHD.
As the field of Digital Therapeutics is growing, collaborations between digital therapeutic companies, technology and service providers, pharmaceutical manufacturers, academic institutions, and provider groups can lead to the creation of local, regional, and national roadmaps to better operationalize and commercialize digital therapeutics.
Downloads
Article in PDF
Recent Articles
- Inspira™ Approval of INSPIRA™ ART100 System; Oticon Medical’s Sentio™ System Received Regul...
- How Metaverse is Set to Transform the Healthcare Dynamics?
- Role of Digital Therapeutics (DTx) in Mental Health Management
- Relievant Medsystems Launched Ablation System; Thermo Fisher’s Reproductive Health Assays; EarliT...
- Watch Out For Top Pipeline Therapies Making An Impact In The Bipolar Depression Market


