Is the Cure for Duchenne Muscular Dystrophy in the Pipeline?
Aug 20, 2024
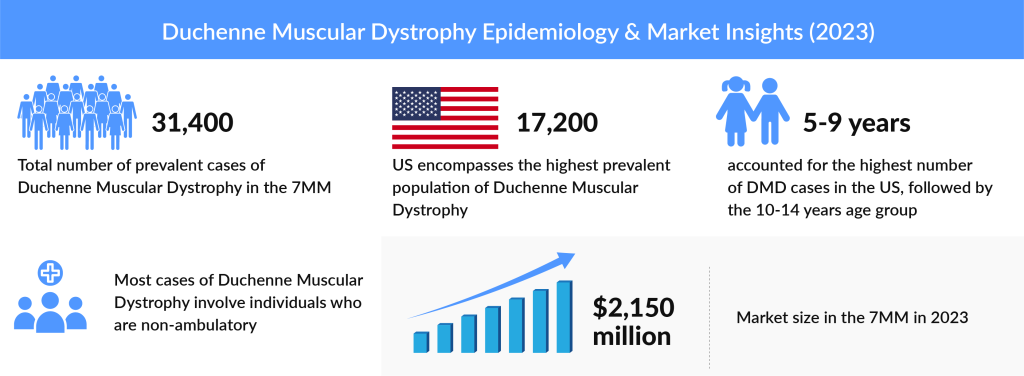
A rare muscle disorder, Duchenne Muscular Dystrophy (DMD), approximately affects 1 in 3,500 male births globally. In 2023, the total number of prevalent cases of Duchenne Muscular Dystrophy in the 7MM (The US, EU5, and Japan) was around 31,400. The United States encompasses the highest prevalent population of Duchenne Muscular Dystrophy, with approximately 17,200 cases in 2023. In the US, the age group of 5-9 years accounted for the highest cases in 2023, followed by the 10-14 years age group.
Duchenne Muscular Dystrophy is an X-linked genetic disorder caused by a mutated gene on the X-chromosome, hence the reason why it mostly manifests in males. Females with the defective gene become the carriers hence passing the gene forward to next generation. The mutation in the DMD gene which is responsible for transcribing into a protein called dystrophin, which plays an essential role in maintaining the integrity of cell membrane in skeletal and cardiac muscle cells. Absence of protein, over time, leads to degeneration of muscle fibres, a progressive weakening of muscles and is known to affect communication, and socio-emotional skills as well. Shockingly, by the age children turn 13, they become dependent on wheelchairs.
Duchenne Muscular Dystrophy Market: Unmet Needs and Hope for a Cure
For such a disabling disease, the goal of the treatment of the disease until now remains symptomatic in nature by managing the associated complication and maximize the quality of life.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Venclexta full approval; PBP1510 Orphan Status; Roche/ Genesis collab; Immunomodulators for COVID-19
- Sanofi to Acquire Provention Bio; USFDA Committee Votes in Favor Roche’s Polivy; FDA to Rev...
- PMV Raises; Argos Shares Plummet; Cidara Flunks in; Sarepta Sells to Gilead; Novartis and NHS Ayr...
- PD-1 and PDL-1 Immune Check Point Inhibitors: A Prodigious Revolution in Cancer Treatment
- Dubai: A leading and exciting pharma hotspot
Several forms of therapies such as Corticosteroids, Steroids, Assistive devices, Physical therapies, and others are available in the market that helps in alleviating the symptoms. Corticosteroids, so far, have remained the standard treatment and help in strengthening muscles and improving ling function. Assistive devices help the patients with breathing difficulties, on the other hand, Orthopedic devices help in facilitating the movements of the patients. Steroids have also proven to be beneficial in extending the ability of the patient to walk by 2–5 years; however, use of steroids can result in side effects such as weight gain, high blood pressure, behavior changes, and delayed growth. Some of the available steroids in the Duchenne Muscular Dystrophy Market are Prednisone, Deflazacort, and Oxandrolone. Some of the medications that have been granted orphan drug status are Deflazacort, manufactured by PTC Therapeutics/Marketed by Marathon Pharmaceuticals is FDA-approved treatment for Duchenne Muscular Dystrophy in patients 5 years of age and older. Other than this, In June 2024, Cranbury Pharmaceuticals received FDA approval for the generic version of EMFLAZA (deflazacort) for DMD. Whereas, Eteplirsen, manufactured by Sarepta Therapeutics, is approved for treating DMD in patients with a confirmed mutation of the DMD gene that is amenable to exon 51 skipping. In March 2024, a study titled “Survival among patients receiving eteplirsen for up to 8 years for the treatment of Duchenne muscular dystrophy” highlighted improved survival rates for eteplirsen-treated DMD patients, with a median survival age of 32.8 years compared to 27.4 years in natural history controls.
In addition to Deflazacort and Eteplirsen, Ataluren is approved for use in ambulatory patients aged two years and older with nmDMD within the European Union (EU), Iceland, Liechtenstein, Norway, Israel, and South Korea. The current US market includes approved products such as EMFLAZA (deflazacort), VYONDYS 53 (golodirsen), EXONDYS 51 (eteplirsen), AMONDYS 45 (casimersen), VILTEPSO (viltolarsen), and ELEVIDYS (delandistrogene moxeparvovec) for treating patients with Duchenne Muscular Dystrophy.
However, the current Duchenne Muscular Dystrophy Market is evaluating the potential of genetically engineered therapies, including exon skipping, the use of recombinant adeno-associated virus carrying mini-dystrophin, and surrogate gene transfer, to be incorporated into the DMD pipeline. Moreover, extensive research is underway tracking the role of palliative care in improving the course of the disease during adolescence and beyond.
At present, it is one of the major unmet needs and calls for a proper understanding of the concept of adolescence and neuromuscular conditions as Duchenne Muscular Dystrophy prevalence keeps on rising. Furthermore, the challenges that obstruct the successful delivery of gene therapies such as the product gene size, the origin of dystrophin gene expression, transport effectiveness, and immunological rejection needs to be addressed to tackle the burden DMD is posing.
To address various such prevailing unmet needs in the Duchenne Muscular Dystrophy Market, several randomized trials are being conducted by pharma and biotech companies that are currently researching new drugs with novel mechanisms of action such as drugs with a high-efficacy/toxicity ratio to improve outcomes in DMD. companies like FibroGen, Santhera Pharmaceutical, Italfarmaco, ReveraGen BioPharma, Cumberland Pharmaceuticals, Sarepta Therapeutics, Antisense Therapeutics, Capricor Therapeutics, and others are working toward to accelerate the Duchenne Muscular Dystrophy landscape.
Some of the companies have recently found the DMD market prosperous enough to invest their time and money in it; however, others have long been working to advance the DMD treatment market and find a standard cure for the disease. In addition to ongoing research activities and a rich Duchenne Muscular Dystrophy pipeline, an increasing proportion of the disease among adolescents would also influence the Duchenne Muscular Dystrophy Market during the forecast period.

Downloads
Article in PDF
Recent Articles
- NIH awards $107M for COVID-19 testing tech; GSK secures rights; Roche’s bispecific matches ...
- Mylan closing Illinois plant; Insys spends $24M; Roche’s Ocrevus; Greece’s corruption prose...
- Next Generation Antibody-Drug Conjugate Therapeutics and Market Analysis
- The Business Cocktail : Latest Pharma deals
- Notizia



