Dupixent Breaks Ground: First and Only Eosinophilic Esophagitis Treatment for Pediatric Patients
Feb 09, 2024
Sanofi and Regeneron are prioritizing pediatric care, particularly in their recent progress with the potent anti-inflammatory drug, Dupixent. On January 25, 2024, the FDA approved Dupixent, an IL-4 receptor alpha antagonist, for the treatment of eosinophilic esophagitis (EoE) in children aged 1 to 11, weighing at least 33 pounds. This marks Dupixent as the inaugural treatment in the United States for youngsters within this age range who are affected by the disorder.
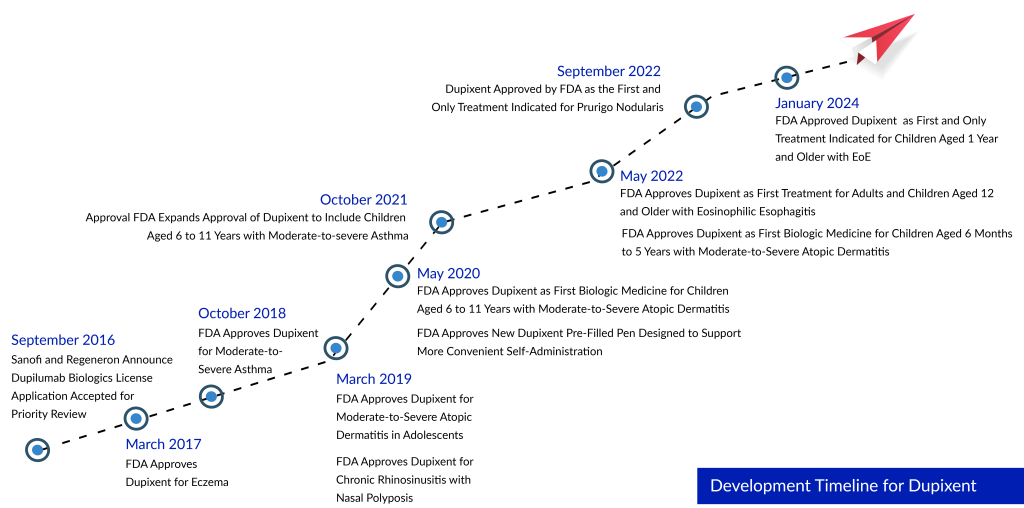
This approval broadens the original FDA approval granted in May 2022 for EoE in individuals aged 12 years and above, with a minimum weight of 40 kg. Dupixent underwent Priority Review by the FDA for this extended use, a designation reserved for medications that may bring substantial advancements in effectiveness or safety for treating severe conditions.
Eosinophilic esophagitis is an enduring and advancing condition linked to type 2 inflammation, believed to be the cause of harm to the esophagus and disruption of its normal function. It can significantly impact a child’s ability to eat, manifesting as heartburn, vomiting, abdominal discomfort, difficulty swallowing, food refusal, and failure to thrive. These symptoms can negatively affect a child’s growth and developmental milestones. Consistent management of EoE may be necessary to minimize the risk of complications and the progression of the disease. Currently, around 21,000 children under the age of 12 in the US are undergoing treatment for EoE with therapies that have not received official approval. However, the actual prevalence of this disease among children is likely higher, as its symptoms can be confused with other conditions, leading to delays in diagnosis.
Downloads
Article in PDF
Recent Articles
- Can Dupixent Be A Gamechanger In The Atopic Dermatitis Treatment Landscape?
- Sanifit nets USD 80.9 M; FDA approves Dupixent for CRS; AbbVie buys Allergan
- Precigen’s PRGN-3006; Yescarta Approved as a First CAR T-cell Therapy for R/R LBCL; Biogen ...
- How Emerging Pipeline Therapies Will Unfold the Severe Asthma Treatment Market Dynamics?
- ATS 2023 Updates: Dupixent – A Ray of Hope For Moderate To Severe Chronic Obstructive Pulmo...

DelveInsight’s analysis reveals that the overall prevalent population of EoE in the 7MM was reported as ~700K in 2021. As per the estimates, the US had the highest patient population of eosinophilic esophagitis in 2021. Among the EU5 countries, Germany had the highest diagnosed patient population of Eosinophilic Esophagitis, with approximately 58K cases, followed by France in 2021.
“Young children suffering from eosinophilic esophagitis face significant vulnerability, as this debilitating and progressive disease poses a threat to their fundamental ability to eat. Up until now, there were no approved treatment options specifically designed for EoE in these children. Many resorted to using unapproved medications that did not address the root cause of their condition,” stated Dr. George D. Yancopoulos, Co-Chair of the Board, President, and Chief Scientific Officer at Regeneron, and a key inventor of Dupixent. “With the recent approval, Dupixent emerges as the inaugural and sole treatment choice for EoE patients aged 1 and older, weighing at least 15 kg. By targeting the underlying type 2 inflammation contributing to this disease, Dupixent holds the potential to revolutionize the standard of care for these children, much like it has for adults and adolescents grappling with EoE.”
Dupixent has been authorized for the treatment of five inflammatory conditions, and efforts have been made by the companies to broaden its application to pediatric patients. In June 2022, the FDA approved Dupixent to be used in children aged 6 months to five years suffering from atopic dermatitis (eczema). Additionally, Sanofi and Regeneron received approval for the use of Dupixent in children aged 6 to 11 with asthma. Dupixent has received approval in over 60 nations, with Regeneron and Sanofi collaborating on the development of Dupilumab through a global partnership established in January 2018. Presently, over 190,000 patients worldwide have undergone treatment.
For children dealing with EoE, managing the condition poses significant difficulties. They need to be cautious about their dietary choices, especially when it comes to consuming foods that may trigger inflammation in the esophagus, such as milk, eggs, and wheat. This disorder can adversely affect their growth, weight gain, and overall development. Additionally, it can instill a lasting sense of food-related anxiety that may persist into adulthood.
FDA approval for Dupixent in children aged 1 to 11 years with EoE is supported by Phase III EoE KIDS trial data (Parts A and B). The trial evaluated Dupixent’s efficacy and safety, using tiered dosing regimens based on weight. At 16 weeks, 66% of children on higher dose Dupixent achieved histological disease remission (≤6 eosinophils/high power field), the primary endpoint, compared to 3% on placebo. Histological remission persisted at week 52, with 53% of children treated with Dupixent in Parts A and B maintaining remission. Additionally, 53% of children who switched to Dupixent from placebo in Part B achieved histological remission at week 52. Moreover, children on Dupixent showed a greater reduction in the proportion of days with EoE signs/symptoms at 16 weeks compared to those on placebo, based on the Pediatric EoE Sign/Symptom Questionnaire-caregiver version (PESQ-C).
“Children suffering from eosinophilic esophagitis face substantial healthcare challenges. Despite available treatment choices, 40% of those under 12 years old in the U.S. still endure symptoms of this condition,” remarked Dr. Naimish Patel, Head of Global Development, Immunology, and Inflammation at Sanofi. “This approval affirms our dedication to providing solutions for young patients with unmet needs, offering hope to those navigating a critical period where difficulties in eating and maintaining weight directly influence their overall nutritional well-being and development.”
The safety profile of Dupixent, as assessed over a 16-week period in children between the ages of 1 and 11 years, weighing at least 15 kg, were generally comparable to the safety profile observed over 24 weeks in both adult and pediatric patients aged 12 years and above with EoE. The prevalent adverse events (occurring at a frequency of ≥2%) more commonly associated with Dupixent compared to a placebo included injection site reactions, upper respiratory tract infections, joint pain (arthralgia), and herpes viral infections. In the EoE KIDS Part B study, a single instance of helminth infection was reported in the group receiving Dupixent.
Dupixent first gained approval from the FDA for atopic dermatitis in 2017. Subsequent approvals were granted for asthma in 2018, rhinosinusitis with nasal polyps in 2019, and prurigo nodularis in 2022. With two phase III trials demonstrating success, Dupixent is poised for FDA approval to address COPD. In 2022, Dupixent generated sales amounting to $8.7 billion, and it is anticipated that annual global sales will reach $20 billion by the close of the decade.
According to DelveInsight’s analysis, the market size for EoE reached USD 600 million in 2021 across the 7MM and is expected to grow with a significant CAGR by 2032. This is mainly due to the launch of new therapies in the market that will give stiff competition to Dupixent and the rise in the number of cases, along with the rising healthcare spending across the globe.
The Dupixent manufacturer Regeneron employed VelocImmune technology in the creation of the Dupixent medication. This technology utilizes a unique genetically engineered mouse platform equipped with a humanized immune system to generate highly efficient fully-human antibodies.
Regeneron and Sanofi are collaborating globally on the development of Dupilumab. Dupixent has undergone scrutiny in over 60 clinical trials, encompassing more than 10,000 patients dealing with various chronic illnesses, primarily influenced by type 2 inflammation. Beyond the presently approved indications, the companies are exploring Dupilumab’s effectiveness in Phase III trials for diverse conditions linked to type 2 inflammation or other allergic processes. These include chronic pruritus of unknown origin, chronic obstructive pulmonary disease (COPD) displaying evidence of type 2 inflammation, and bullous pemphigoid. It’s important to note that these potential applications of Dupilumab are currently in clinical investigation, and regulatory authorities have not yet fully assessed their safety and efficacy.

Downloads
Article in PDF
Recent Articles
- Atopic dermatitis market and a Dupilumab dominance
- The Evolving Landscape of COPD Treatments: New Hope for Patients
- Is it the Dawn of Biologics in the Eosinophilic Esophagitis Treatment Landscape?
- How Emerging Pipeline Therapies Will Unfold the Severe Asthma Treatment Market Dynamics?
- Towards a Promising Future: Unveiling Advancements in Chronic Spontaneous Urticaria (CSU) Treatment