Can Dupixent Be A Gamechanger In The Atopic Dermatitis Treatment Landscape?
Mar 25, 2022
Dupixent (dupilumab) is a non-immunosuppressive fully human monoclonal antibody that inhibits the signalling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) proteins. According to data from Dupixent clinical trials, IL-4 and IL-13 are key drivers of type 2 inflammation, which is a major contributor to Atopic Dermatitis, Asthma, and Chronic Rhinosinusitis with Nasal Polyposis (CRSwNP).
There are two Dupixent dosings available (200 mg and 300 mg), each in the form of a pre-filled syringe. After an initial loading dosage, Dupixent is given every other week through an injection beneath the skin (subcutaneous Dupixent injection). It can be self-administered in a clinic or, for convenience, at home by self-administration after training by a healthcare professional. Dupixent commercially available globally, including in the US.
The most common Dupixent side effects are injection site reactions, throat pain (oropharyngeal pain), and cold sores in the mouth or on the lips. In addition, the other side effects of Dupixent that Atopic Dermatitis patients suffer include eye and eyelid inflammation, including redness, swelling, and itching. Despite that, there are positive Dupixent reviews for the Atopic Dermatitis treatment by the users.
Downloads
Article in PDF
Recent Articles
- GBT strikes a deal with Sanofi; Lilly’s IL-23 inhibitor shows promise; Glooko raises $30M; ...
- Immune cells for age-related diseases; Regeneron’s antibody for 2019-nCoV; FDA nods for hyd...
- Toujeo to treat Type 1 Diabetes; TauRx for Alzheimer’s patients
- Johnson & Johnson’s TREMFYA Approved for Ulcerative Colitis; Roche’s Tecentriq Hybreza Approv...
- Immutep’ First-Line Treatment Positive Outcomes; Pfizer’s Once-Daily Oral GLP-1 Agonist Danuglipr...
The Dupixent manufacturer Regeneron used VelocImmune technology to develop Dupixent drug that utilizes a proprietary genetically engineered mouse platform endowed with a genetically-humanized immune system to produce optimized fully-human antibodies.
Dupixent has been approved for adolescents and adults with moderate-to-severe Atopic Dermatitis in several countries around the world, including the US, where Dupixent is also approved for children with moderate-to-severe Atopic Dermatitis. The other Dupixent indications are Asthma and Chronic Rhinosinusitis with Nasal Polyposis (CRSwNP).
Dupixent is currently approved in over 60 countries. Regeneron and Sanofi are jointly developing Dupilumab under a global collaboration agreement since January 2018. Currently, more than 190,000 patients have been treated globally. Moreover, in 2021 the total prevalent population of Atopic Dermatitis was more than 65 million in the 7MM. Furthermore, the rising Atopic Dermatitis prevalence is anticipated to drive market growth. As per the DelveInsight Atopic Dermatitis Market Report, the Atopic Dermatitis market size was USD 15,026 million in the 7MM in 2021, which is further expected to increase by 2032.
Recently there is a Dupixent update for Atopic Dermatitis. In February 2022, the FDA has accepted the supplemental Biologics License Application for Dupixent (dupilumab) for Priority Review as an add-on maintenance treatment for children aged 6 months to 5 years with moderate-to-severe Atopic Dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. The FDA’s decision on this investigational use is expected to be made on June 9, 2022. Dupixent is the only biologic medicine approved for patients 6 years of age and older in this indication. The sBLA is supported by the data from a pivotal Phase 3 trial examining the effectiveness and safety of Dupixent in conjunction with standard-of-care topical corticosteroids (TCS) in children aged 6 months to 5 years with uncontrolled moderate-to-severe Atopic Dermatitis. Additionally, the company is also conducting a phase III trial in Japanese pediatric patients with Alzheimer’s disease (aged 6 months to 18 years old).
In May 2020, the FDA approved Dupixent (dupilumab) for children aged 6–11 years with moderate-to-severe Atopic Dermatitis whose disease is not adequately controlled by topical prescription therapies or when those therapies are contraindicated. The FDA approval of Dupixent use was based on pivotal Phase III results comparing the efficacy and safety of Dupixent combined with topical corticosteroids (TCS) to TCS alone in children with severe Atopic Dermatitis.
In June 2020, the FDA approved a Dupixent 300 mg single-dose pre-filled pen. The pre-filled Dupixent pen is approved for all Dupixent indications in patients aged 12 years and older, including at-home administration in certain patients with Atopic Dermatitis, Asthma, and Chronic Rhinosinusitis with Nasal Polyposis (CRSwNP). This new pre-filled pen will make Dupixent administration more convenient for patients.
In November 2020, the European Commission (EC) extended the marketing authorization for Dupixent (dupilumab) in the EU to include children aged 6 to 11 with severe Atopic Dermatitis who are candidates for systemic therapy. The EC decision is primarily based on data from pivotal Phase 3 efficacy and safety studies of Dupixent in combination with topical corticosteroids (TCS) versus TCS alone (placebo) in children aged 6 to 11 years with severe Atopic Dermatitis.
It is important to note that prior to the approval of dupilumab, systemic corticosteroids were the only FDA-approved systemic treatment for Atopic Dermatitis in the US. As systemic corticosteroids are not recommended for long-term use, currently, dupilumab is recommended as a first-line systemic treatment option for patients with moderate-to-severe Atopic Dermatitis.
Dupixent (dupilumab) is a non-immunosuppressive completely human monoclonal antibody that inhibits the signalling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) proteins. Regeneron and Sanofi collaborated on the drug, which is FDA-approved for the treatment of Asthma, Chronic Rhinosinusitis with Nasal Polyposis (CRSwNP), and Atopic Dermatitis. Clinical trial results have demonstrated that three-quarters of patients receiving Dupixent achieved at least a 75% improvement in the overall disease. However, the drug is a little expensive. Dupixent costs around USD 15,000 per syringe, and therefore, physicians first prescribe cheaper drugs before switching to expensive medicine. Additionally, the company reported additional data in patients as young as 6 years old with moderate-to-severe Atopic Dermatitis. DelveInsight expects the Dupixent drug to reach peak sales of approximately USD 4.5 Billion in the United States by the year 2026.
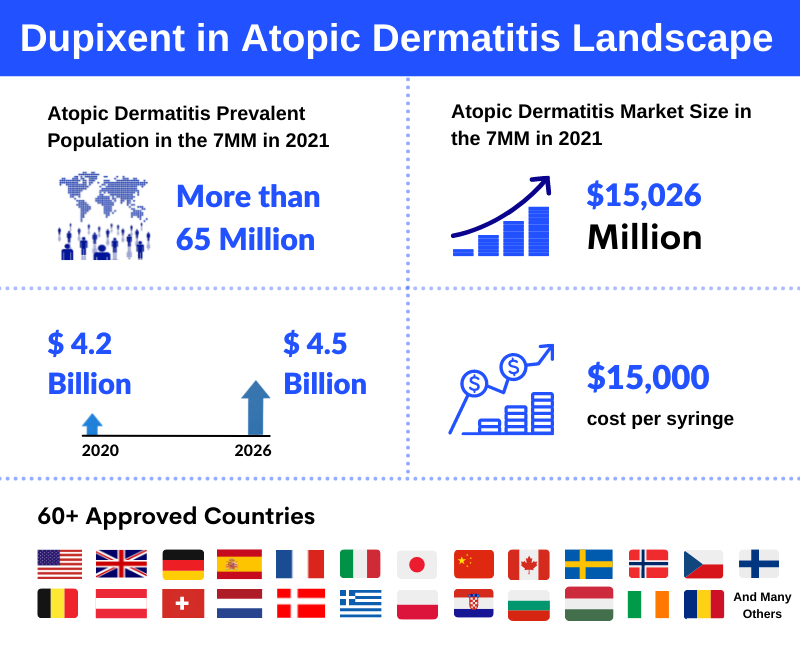
The Dupixent drug is currently considered a blockbuster drug, as for the year 2020, it generated EUR 3.5 billion (about USD 4.2 billion) for Sanofi. The company mentioned that its sales were driven by continued strong demand in Atopic Dermatitis in adults and adolescent patients, rapid adoption in children aged 6–11 years, continued Asthma, and CRSwNP. Furthermore, by the end of 2020, Dupixent was available in 47 different countries, with approximately 230,000 patients on therapy.
The current US market possesses Dupixent, Eucrisa (Crisaborole 2%), Cibinquo, Rinvoq, Adbry (tralokinumab), and Opzelura cream to treat patients with Atopic Dermatitis. Although Dupixent is being evaluated for its safety and efficacy in Atopic Dermatitis patients aged 6 months to 5 years, Eucrisa is a phosphodiesterase 4 inhibitor that is used to treat mild to moderate Atopic Dermatitis in adults and children aged 3 months and older. The drug was initially approved in December 2016, with label expansion in March 2020. Incyte Corporation, in September 2021, announced the US FDA approval of Opzelura (ruxolitinib) Cream, a topical JAK Inhibitor, for the treatment of Atopic Dermatitis. It is the first, and only JAK inhibitor approved in the country. In Phase III studies, the topical treatment significantly reduced the skin inflammation and itch associated with AD.
In EU5 countries, the current market holds Dupixent, Staquis (Crisaborole 2%), Olumiant (Baricitinib), and the recently approved Rinvoq and Adtralza (tralokinumab). Moreover, Cibinqo (abrocitinib) was approved in September 2021 by the UK’S MHRA and EMA for adults and adolescents with moderate to severe Atopic Dermatitis. Apart from these systemic pharmacological treatments, the treatment landscape for patients with Atopic Dermatitis in the EU-5 countries holds the use of off-label therapies similar to the US.
The treatment in Japan for Atopic Dermatitis is similar to that of the US and EU-5. The Japanese market holds the use of Dupixent, Corectim Ointment 0.5% and 0.25%, Difamilast, and Olumiant (Baricitinib). Recently, in September 2021, Cibinqo (abrocitinib), an oral, once-daily, JAK1 inhibitor, was approved in Japan for moderate to severe Atopic Dermatitis treatment in adults and adolescents aged 12 years and older with inadequate response to existing therapies. The country’s therapeutic choices for mild to severe Atopic Dermatitis were restricted in the past. The approval raises the prospect of the Dupixent drug having a positive influence on the lives of people in Japan who suffer from this chronic and possibly debilitating disease.
Conclusively, while treatment of atopic itch is challenging, advancements in the last several years have provided multiple options ranging from topical agents to systemic therapies. In addition, newer treatments on the horizon hold great promise. Moreover, improved treatment of atopic itch will improve not only Atopic Dermatitis symptoms but also the quality of life in many patients across 7MM.
FAQs
Dupixent developed by Sanofi and Regeneron is a fully-human monoclonal antibody that inhibits the signalling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) proteins and is not an immunosuppressant.
Dupixent is currently approved in more than 60 countries. Dupixent has been approved for adolescents and adults with moderate-to-severe Atopic Dermatitis, Asthma and Chronic Rhinosinusitis with Nasal Polyposis (CRSwNP) and for children with moderate-to-severe Atopic Dermatitis.
Several other therapies such as Eucrisa (Crisaborole 2%), Cibinquo, Rinvoq, Adbry (tralokinumab), and Opzelura cream are available in the US market to treat patients with Atopic Dermatitis.
The leading companies currently working in the Atopic Dermatitis market include Sanofi, Regeneron Pharmaceuticals, Pfizer, Japan Tobacco and Torii Pharmaceutical, Eli Lilly and Company, AbbVie, LEO Pharma, Incyte Corporation, Arena Pharmaceuticals, Oneness Biotech, Galderma, DS Biopharma, Janssen, Kymab, Arcutis Biotherapeutics, Qurient, Cara Therapeutics, Vanda Pharmaceuticals, among others.
Downloads
Article in PDF
Recent Articles
- VistaGen’s PH94B for Anxiety Disorder; Keytruda for Head and Neck Cancer Treatment; Bavarian Nord...
- European Commission gives a nod to Zynquista for type 1 diabetes in adults
- 5 Promising Exosome-based Therapies Paving the Way for Personalized Medicine
- Sanofi acquires Principia; FDA’s Nod to Roche’s Enspryng; BMS, Dragonfly’s Lice...
- Business Cocktail