7 Sickle Cell Disease Therapies to Keep an Eye on Post-Pfizer’s OXBRYTA Withdrawal
Jan 03, 2025
In September 2024, Pfizer voluntarily withdrew all lots of OXBRYTA for the treatment of SCD in all markets where it was approved. Pfizer is also discontinuing all active voxelotor clinical trials and expanding access programs worldwide. The decision is based on the totality of clinical data, which now indicates that the overall benefit of OXBRYTA no longer outweighs the risk in the approved sickle cell patient population. The data suggest an imbalance in vaso-occlusive crises and fatal events, which requires further assessment.
OXBRYTA, which received FDA accelerated approval in November 2019, was one of the treatments in question. Another such treatment, Novartis’ ADAKVEO, faced scrutiny after it did not reduce vaso-occlusive crises (VOC) in individuals with sickle cell disease in a Phase III trial last year. While EU regulators officially withdrew ADAKVEO’s approval in August 2023, it is still available in the US.
The future of SCD treatment is rooted in next-generation transplantation and gene therapy. With the approval of bluebird bio’s LYFGENIA and Vertex and CRISPR Therapeutics’ CASGEVY, there is significant potential for gene therapies in SCD. However, the adoption has been gradual, and these treatments remain largely inaccessible for most.
Downloads
Article in PDF
Recent Articles
- DelveInsight’s Hematological disorders based Gene Therapy Reports
- World Sickle Cell Day
- FDA Approves AstraZeneca’s Enhertu; Bayer Wins FDA Approval for Prostate Cancer Therapy, Nubeqa; ...
- Lyfgenia or Casgevy: Who Will Lead the Sickle Cell Disease Treatment Space?
- FDA Grants Orphan Status to MDL-101 for LAMA2-CMD; Pfizer’s ABRYSVO Approved for High-Risk Adults...
Read our article “LYFGENIA or CASGEVY: Who Will Lead the Sickle Cell Disease Treatment Space?” and get a more detailed analysis
Additionally, the sickle cell disease pipeline includes several drugs currently in development that are anticipated to receive approval in the near future. The expected introduction of therapies such as EDIT-301 (Editas Medicine), Inclacumab and Osivelotor (Pfizer), Motixafortide (BiolineRx), Etavopivat (Novo Nordisk), Mitapivat (Agios Pharmaceuticals), and Pociredir (Fulcrum Therapeutics) is likely to significantly influence the SCD treatment market positively.
Now, let’s examine these 7 most promising sickle cell therapies in detail.
Pfizer’s Osivelotor and Inclacumab
OXBRYTA is not the only sickle cell disease treatment Pfizer acquired through its 2022 purchase of Global Blood Therapeutics. The acquisition also included two other experimental therapies, inclacumab and osivelotor.
Inclacumab, a P-selectin inhibitor, is currently being tested in a Phase III trial to evaluate its safety and effectiveness in reducing vaso-occlusive crises (VOC). It works by binding to P-selectin, a cell adhesion molecule on platelets, which prevents platelet aggregation and is expected to help maintain proper blood flow.
Osivelotor, like OXBRYTA, is a sickle hemoglobin polymerization inhibitor but features a next-generation design. It is being studied in a Phase II/III trial, with completion expected in 2028. While Pfizer has not indicated whether recent developments regarding OXBRYTA will influence osivelotor’s progress, Leerink Partners highlighted that a Phase II trial raised safety concerns, with 8 out of 35 patients experiencing treatment-emergent adverse events.
Agios Pharmaceuticals’ Mitapivat
Mitapivat is an innovative, first-of-its-kind oral small molecule that acts as an allosteric activator of the pyruvate kinase enzyme. It has been demonstrated to significantly enhance the activity of both wild-type and various mutant forms of erythrocyte pyruvate kinase (PKR), leading to increased production of adenosine triphosphate (ATP) and reduced levels of 2,3-diphosphoglycerate. In February 2022, the FDA approved PYRUKYND (mitapivat) for treating hemolytic anemia in adults with pyruvate kinase deficiency.
The company has begun a Phase II/III trial to assess mitapivat’s effectiveness in patients with sickle cell disease. In June 2023, Agios Pharmaceuticals revealed that the Phase II portion of the global RISE UP study of mitapivat in sickle cell disease successfully met its primary endpoint, demonstrating a hemoglobin response in patients across both the 50 mg and 100 mg twice-daily (BID) mitapivat doses.
In December 2023, the company shared encouraging results from the Phase II segment of its pivotal RISE UP study. The annualized rate of sickle cell pain crises was 0.51 for patients in the higher-dose treatment group, compared to 1.71 for those in the placebo group.
Novo Nordisk’s Etavopivat
In 2022, Novo Nordisk acquired Forma Therapeutics for $1.1 billion, securing etavopivat, a leading asset for sickle cell disease. Similar to mitapivat, etavopivat is a PK activator, but it offers a potential advantage with once-daily dosing compared to mitapivat’s twice-daily schedule. Etavopivat is currently undergoing a Phase III trial expected to conclude in 2026. The drug has received FTD, RPDD, and ODD by the US FDA for treating SCD.
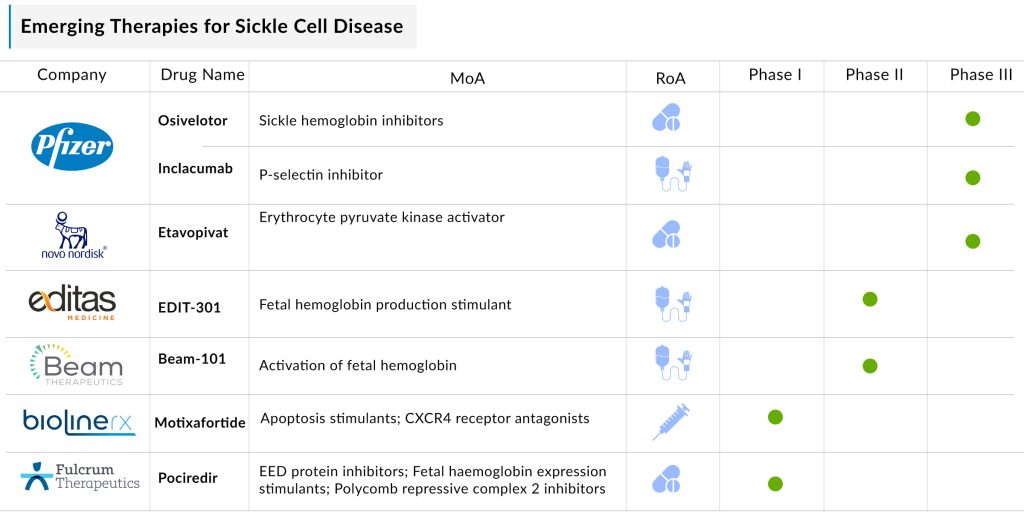
Fulcrum Therapeutics’ Pociredir
Fulcrum Therapeutics’ pociredir, a polycomb repressive complex 2 (PRC2) inhibitor, is designed to boost the production of fetal hemoglobin, compensating for the defective hemoglobin associated with sickle cell disease.
Pociredir’s development journey has faced challenges. In February 2023, the FDA placed a clinical hold on the drug due to preclinical data and evidence of hematological malignancies linked to other PRC2 inhibitors. This hold was lifted six months later.
Currently, in a Phase Ib trial, pociredir has demonstrated good tolerability in individuals with SCD, with no treatment-related adverse events reported after up to three months of use.
Editas Medicine’s EDIT-301
EDIT-301 consists of a patient’s CD34+ hematopoietic stem and progenitor cells, which have been genetically modified using a CRISPR/Cas12a ribonucleoprotein (RNP) to target the HBG1/2 promoter region within the beta-globin locus. In April 2023, the FDA granted Orphan Drug Designation (ODD) to EDIT-301 for the treatment of sickle cell disease, following its earlier designation of Rare Pediatric Disease Designation (RPDD) for the same condition.
The therapy is currently under investigation in a Phase I/II RUBY trial to assess its efficacy, safety, and tolerability in adult and adolescent participants with severe sickle cell disease. Preliminary results from the RUBY trial indicate that EDIT-301 has been well-tolerated and demonstrates promising efficacy in patients with SCD.
Beam Therapeutics’ Beam-101
BEAM-101 is an experimental cell therapy designed to treat severe sickle cell disease. This one-time therapy uses a patient’s own CD34+ hematopoietic stem and progenitor cells (HSPCs), which are genetically modified by base-editing specific regions of the HBG1/2 gene promoters. These modified cells are delivered through a hematopoietic stem cell transplant. The editing process prevents the transcriptional repressor BCL11A from binding to the promoters without affecting its overall expression, thereby boosting the production of fetal hemoglobin (HbF), which is non-sickling and anti-sickling.
This mimics the beneficial effects observed in individuals with hereditary persistence of fetal hemoglobin, where HbF remains the dominant hemoglobin during early life. The safety and effectiveness of BEAM-101 are being assessed in the BEACON Phase I/II trial, a multicenter, single-arm, open-label study involving adult SCD patients experiencing severe vaso-occlusive crises (VOCs).
In December 2024, Beam Therapeutics revealed updated safety and efficacy findings from its BEACON Phase I/II clinical trial of BEAM-101. These results were highlighted during the press program at the 66th American Society of Hematology (ASH) Annual Meeting and Exposition in San Diego.
BiolineRx’s Motixafortide
Motixafortide is an innovative, high-affinity inhibitor of CXCR4. This novel compound targets the CXCR4 receptor, which is expressed in various cell types, including hematopoietic stem cells, immune cells, and cancer cells. Its unique mechanism of action positions it as a promising candidate for multiple applications, such as stem cell mobilization and cancer therapy.
In March 2023, BioLineRx revealed a partnership with Washington University School of Medicine to support a Phase I clinical trial investigating the safety and viability of motixafortide, both as a standalone treatment and in combination with natalizumab (a VLA-4 inhibitor), for mobilizing CD34+ hematopoietic stem cells (HSCs) in gene therapy for patients with sickle cell disease.
In May 2024, BioLineRx revealed a partnership with St. Jude Children’s Research Hospital, Inc. to conduct a multi-center Phase I clinical trial. This study aims to assess the safety, tolerability, and feasibility of using motixafortide as a single agent for mobilizing and collecting CD34+ hematopoietic stem cells in 12 patients aged 18 and older with sickle cell disease.
The expected launch of these sickle cell disease drugs in the coming years will bring stiff competition in the space. Additionally, the future of sickle cell disease treatment, with ongoing research and advancements in understanding disease mechanisms, is promising.

Downloads
Article in PDF
Recent Articles
- Vertex/CRISPR’s Gene-editing Therapy exa-cel: Inch Ahead of Rival
- DelveInsight’s Hematological disorders based Gene Therapy Reports
- FDA Approves AstraZeneca’s Enhertu; Bayer Wins FDA Approval for Prostate Cancer Therapy, Nubeqa; ...
- Sickle Cell Disease Treatment: How Gene Therapy and Editing Could Transform Therapeutic Segment?
- Merck and Moderna Initiate Study to Evaluate V940; FDA Approves Vertex and CRISPR Therapeutics’ C...