Advances in Non-Muscle Invasive Bladder Cancer Treatment: Exploring Emerging Therapies
Apr 01, 2024
Non-muscle-invasive bladder cancer represents a category of bladder cancer where the tumor is confined to the innermost layer of the bladder lining without invading the muscle. This early-stage form accounts for a significant proportion of bladder cancer cases.
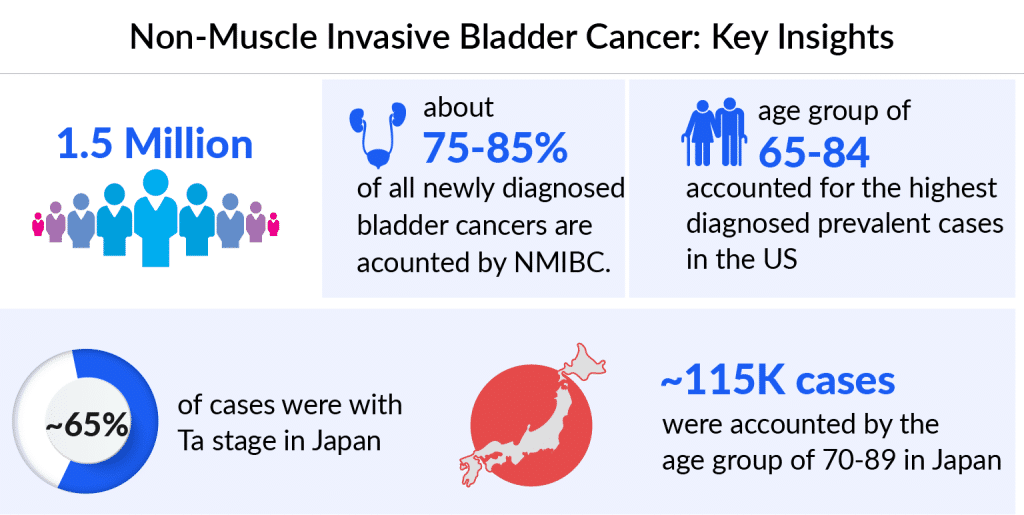
Among the 7MM, the US accounted for the highest number of NMIBC cases in 2023, with around 600K prevalent cases, these cases are expected to increase during the forecast period, as per DelveInsight. Among cases categorized by risk level, the highest number belonged to the intermediate-risk category. According to the estimates, in Japan, it is observed that NMIBC was most prevalent in the 70-89 age group, accounting for approximately 60% of total NMIBC cases in 2023.
Patients with high- or intermediate-risk non-muscle-invasive bladder cancer have seen limited advancement in the treatment landscape over the last 50 years and the available options are associated with a high risk of recurrence and significant side effect burden.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- FDA Approves GSK’s Arexvy for RSV; CHMP’s Opinion on Gilead’s Hepcludex® for HDV; FDA Clearance t...
- OPDIVO Approved for Resectable NSCLC; Palvella Gets $2.6M FDA Grant; First Patient Dosed in UGN-1...
- AZ dragout Entasis; Sierra remunerates Gilead; Principia raises; Harbour BioMed and Kelun pen deal
- Roche’s TECENTRIQ HYBREZA: Setting New Standards as the First Subcutaneous Anti-PD-(L)1 Drug
- Fourth FDA Approval for AbbVie’s Vraylar; FDA Approves Ferring’s Adstiladrin for NMIBC; Merck and...
As patients frequently need extended BCG maintenance treatment and continuous monitoring for disease recurrence and advancement, the economic impact on healthcare systems is substantial. The current NMIBC treatment plan involves surgery, intravesical immunotherapy (BCG), and intravesical chemotherapy. For NMIBC of intermediate or high risk, the typical approach is to start with TURBT, followed by BCG immunotherapy as an additional treatment. This regimen is considered the most effective standard for decreasing tumor recurrence rates and halting the progression of subsequent stages. In the case of Stage 0 bladder cancer, the usual course involves TURBT with fulguration, followed by intravesical therapy within 24 hours. In some instances, no further treatment is necessary. Patients then undergo cystoscopy every 3–6 months to monitor for any signs of cancer recurrence.
The treatment choices for NMIBC are quite limited, primarily revolving around VALSTAR, KEYTRUDA, and ADSTILADRIN. Due to the lack of diverse options and the moderate effectiveness of VALSTAR, the FDA took action by guiding to encourage the development of medications for BCG-unresponsive NMIBC cases. This led to the approval of KEYTRUDA in January 2020, followed by ADSTILADRIN in December 2022.
ADSTILADRIN, designed for adult individuals with BCG-unresponsive NMIBC, is a gene therapy. It employs a non-replicating adenovirus vector to deliver the interferon alfa-2b gene. This therapy is introduced into the bladder via catheter, with treatments occurring every three months. Recognized by the European Medicines Agency as an Advanced Therapy Medicinal Product (ATMP), this drug represents an innovative approach to treating this condition.
Pembrolizumab, the primary component of KEYTRUDA, is a humanized monoclonal antibody. It works by attaching to the programmed cell death-1 (PD-1) receptor, preventing its interaction with PD-L1 and PD-L2. This action helps to free the PD-1 pathway, removing the inhibition of the immune response, particularly the body’s ability to mount an antitumor immune reaction.
Given the increased presence of PD-L1 expression in BCG-unresponsive NMIBC patients, which is linked to resistance mechanisms related to adaptive immune responses, much of the research has focused on anti-PD-1/PD-L1 immune checkpoint inhibitors for these individuals. As a result, there is an urgent need for more innovative treatments within the NMIBC treatment landscape.
The emerging NMIBC pipeline for therapies developed to treat NMIBC patients includes late-stage, mid-stage, and early-stage drugs in different therapies. The potential NMIBC drugs that can mark a significant change in the upcoming forecast period include ALT-803, OPDIVO, UGN-102, sasanlimab, CG0070, IMFINZI, ruvidar (TLD-1433), EG-70, erdafitinib, and others being evaluated at different stages of clinical development, respectively.
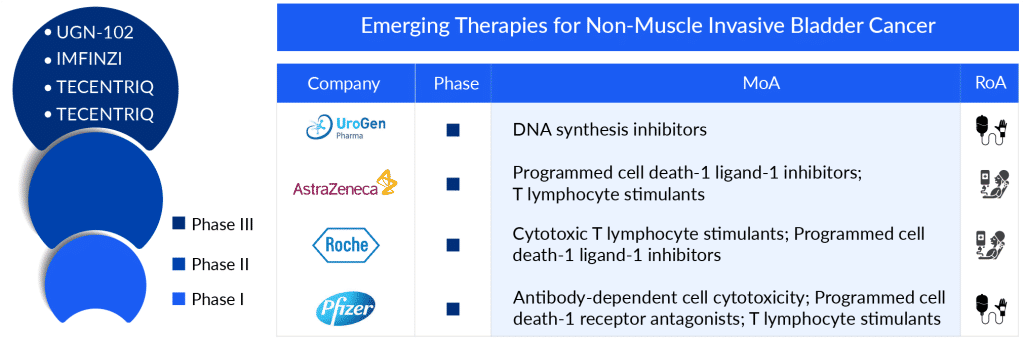
CG0070 (cretostimogene grenadenorepvec) is an oncolytic immunotherapy derived from a modified adenovirus type 5 framework. It features a cancer-specific promoter and includes a GM-CSF transgene. This treatment targets bladder tumor cells by exploiting their faulty Rb pathway. Additionally, CG0070 is being studied in collaboration with Merck to assess its effectiveness when combined with the anti-PD-1 therapy, KEYTRUDA (pembrolizumab), in a Phase II clinical trial for high-grade NMIBC patients who do not respond to BCG treatment.
As of December 2023, CG Oncology has received both Fast Track Designation (FTD) and Breakthrough Therapy Designation (BTD) from the FDA for cretostimogene grenadenorepvec. These designations apply to its use in high-risk BCG-unresponsive NMIBC cases, including those with carcinoma in situ, with or without Ta or T1 (papillary) tumors.
Sasanlimab functions as an anti-PD-1 therapy, working to inhibit the PD-1 protein found on the surface of immune T-cells which might otherwise target healthy cells. PD-1, an immune checkpoint protein, serves to deter T-cells from attacking healthy cells. Healthy cells naturally produce a protein known as PD-L1, which binds to PD-1, effectively deactivating the T-cells. This treatment is classified as an immunotherapy for various forms of cancer.
According to Pfizer’s second-quarter report for 2023, the company is looking ahead to a potential release of sasanlimab for NMIBC post-2024. Presently, the drug is undergoing investigation in a Phase III clinical trial.
UGN-102 (mitomycin) in an intravesical solution is a new form of mitomycin currently in Phase III development for treating LG-IR-NMIBC. This formulation employs UroGen’s specialized RTGel technology, a hydrogel-based system that slowly releases the drug. The aim is to extend the exposure of bladder tissues to mitomycin, allowing for non-surgical tumor treatment. Patients receive UGN-102 via a standard urinary catheter in an outpatient setting. UroGen plans to apply for approval (NDA) for UGN-102 in 2024. The NDA submission will be supported by assessing how long complete response (CR) lasts, measured at 12 months post-treatment from the pivotal ENVISION trial, for the treatment of low-grade intermediate-risk NMIBC.
The anticipated launch of these emerging therapies for NMIBC are poised to transform the NMIBC market landscape in the coming years. These innovative therapies, ranging from immune checkpoint inhibitors to novel intravesical agents, are offering unprecedented hope for improved outcomes and quality of life. By targeting specific pathways and mechanisms, they hold the promise of more effective tumor control, reduced recurrence rates, and potentially even the avoidance of radical surgeries such as cystectomy. This evolving arsenal of treatments not only expands the choices for patients but also presents a paradigm shift in the approach to managing NMIBC. With enhanced efficacy, better tolerability profiles, and the potential for personalized treatment strategies, these emerging therapies are poised to bring about a transformative impact on the NMIBC treatment market, paving the way for more tailored, effective, and patient-centric care.
Managing NMIBC patients who do not respond to BCG treatment in actual clinical settings shows important patterns. Around 25% of doctors’ cases involved patients with NMIBC who did not respond to BCG, meaning BCG therapy was no longer an option for them. Additionally, roughly 1 in 3 NMIBC patients had not received BCG therapy, possibly because they were awaiting treatment after TURBT. The global shortage of BCG has made these issues more difficult, reducing the availability of proper initial and ongoing treatments and leading to higher rates of recurrence and treatment failure.
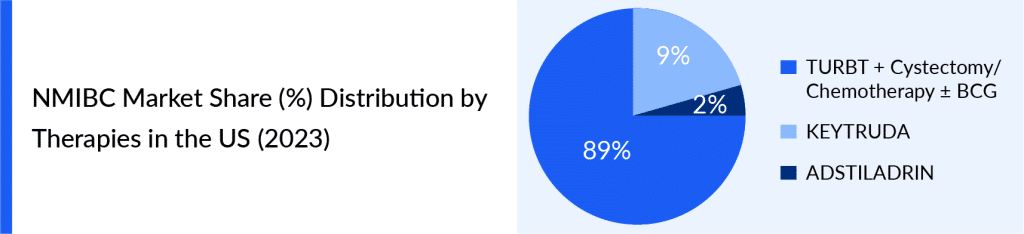
This changing landscape highlights the difficulties and approaches to managing NMIBC patients who do not respond to BCG, underlining the need for more research, personalized treatment plans, and wider use of biomarker-guided methods to enhance patient outcomes.
As per DelveInsight analysis, the total NMIBC market size in the US was estimated to be USD 1.7 billion in 2023, which is expected to grow during the forecast period (2024–2034). Globally, various strains of BCG vaccine, including TICE, Connaught, Tokyo, and Russian strains, are employed. However, in Japan, the Tokyo strain holds prominence as the most commonly utilized BCG strain.
As research and development efforts continue to advance, the NMIBC treatment market is poised for a shift towards personalized medicine, where treatments are tailored to individual patients based on genetic, molecular, and immunological profiles. This trend is expected to lead to the introduction of targeted therapies and immunotherapies, offering more effective and less invasive options for patients. Additionally, the rise of liquid biopsy techniques holds the potential to revolutionize NMIBC diagnostics, enabling early detection, monitoring of treatment response, and recurrence surveillance with greater precision. With a growing emphasis on patient-centric care and the integration of digital health solutions, the future NMIBC treatment market is set to witness a paradigmatic change, offering improved outcomes and enhanced quality of life for those affected by this challenging condition.

Downloads
Article in PDF
Recent Articles
- FDA Approves GSK’s Arexvy for RSV; CHMP’s Opinion on Gilead’s Hepcludex® for HDV; FDA Clearance t...
- Imbalance Between BCG Supply and Increased Demand: The Crisis Impacting Bladder Cancer Treatment
- OPDIVO Approved for Resectable NSCLC; Palvella Gets $2.6M FDA Grant; First Patient Dosed in UGN-1...
- CG Oncology Teams Up With Merck & BMS; Parse Biosciences Partners With Molecular Diagnostics...
- Fourth FDA Approval for AbbVie’s Vraylar; FDA Approves Ferring’s Adstiladrin for NMIBC; Merck and...