Neurotech’s ENCELTO Makes History as the First and Only FDA-Approved Treatment for MacTel
Mar 21, 2025
Neurotech Pharmaceuticals has achieved a significant milestone with the recent FDA approval of its encapsulated cell therapy implant, revakinagene taroretcel, branded as ENCELTO. Approved on March 6, 2025, ENCELTO is the first treatment authorized by the FDA for macular telangiectasia (MacTel) type 2, a rare neurodegenerative eye disorder that leads to vision loss. This approval paves the way for Neurotech’s commercial entry into the MacTel therapeutics market.
MacTel is a neurodegenerative retinal disease in adults that leads to progressive and irreversible vision loss, significantly affecting patients’ quality of life. Macular telangiectasia type 2, also known as idiopathic macular telangiectasia type 2, is a bilateral neurodegenerative disease in adults characterized by localized retinal degeneration.
This condition leads to the gradual loss of retinal cells, resulting in vision impairment and secondary changes in the retinal vasculature — the network of blood vessels that supplies oxygen and nutrients to the retina. MacTel type 2 primarily affects middle-aged adults and typically impacts both eyes, though not always equally. It can also lead to loss of detailed vision.
Downloads
Article in PDF
ENCELTO, developed by Neurotech, addresses MacTel using the company’s proprietary encapsulated cell therapy (ECT) platform, which delivers therapeutic doses of ciliary neurotrophic factor directly to the retina to help slow disease progression.
Neurotech’s ECT platform is a cell-based gene therapy system designed to deliver therapeutic proteins continuously for the long-term treatment of chronic eye diseases. The therapy involves an implant that is surgically placed in the vitreous of the affected eye. It contains 200,000 to 440,000 allogeneic retinal pigment epithelial (RPE) cells that have been genetically modified to produce recombinant human ciliary neurotrophic factor (CNTF).
After surgical implantation, the implant’s semipermeable membrane allows nutrients to enter while releasing therapeutic proteins into the eye, where they can migrate to the retina. The membrane also shields the encapsulated RPE cells from the host’s immune response, supporting their long-term survival and sustained protein production.
ENCELTO offers a significant advantage in convenience over other eye disorder treatments, which typically require repeated injections or refillable implants. Currently, ENCELTO is the only approved treatment for MacTel type 2.
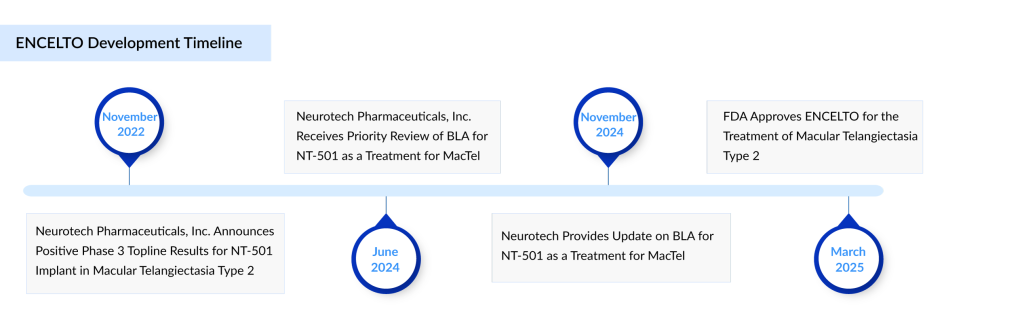
The FDA approved ENCELTO based on data from two late-stage trials, which showed that the treatment significantly slowed the loss of macular photoreceptors in MacTel patients over a 24-month period. In two phase III trials reported in late 2022, Neurotech revealed that ENCELTO—previously identified as NT-501—reduced the rate of MacTel progression by 56.4% and 29.2% compared to a simulated treatment over 24 months.
“This is a remarkable achievement for patients, the retina community, and Neurotech,” said Richard Small, Chief Executive Officer. “I want to thank the clinical trial participants, investigators, and their teams, and the entire Neurotech team for making this possible.”
Dr. Charles C. Wykoff, MD, PhD, of Retinal Consultants of Texas in Houston, TX, and a clinical investigator, shared his optimism: “Having witnessed the impact of MacTel on patients’ lives, I am confident that ENCELTO, as an FDA-approved treatment, will significantly slow disease progression and help patients maintain better functional vision over time.”
The application was reviewed under a priority process, with an initial decision expected in December 2024. However, the FDA extended the review period by about three months to evaluate additional data submitted by Neurotech. ENCELTO is expected to be available to US patients starting in June 2025.
Thomas M. Aaberg Jr, MD, Chief Medical Officer, highlighted the significance of the approval: “ENCELTO’s FDA approval marks a historic moment for the MacTel community. For those affected by this vision-threatening disease and everyone who supported this effort, today marks the beginning of a future where MacTel-related vision loss may finally be slowed.”
During the MacTel clinical trials, the most common side effects reported included conjunctival hemorrhage, delayed dark adaptation, foreign body sensation, and eye pain. ENCELTO’s label also carries warnings about potentially serious complications, including severe vision loss, infectious endophthalmitis, retinal tears or detachment, vitreous hemorrhage, implant extrusion, and cataract formation following implantation.
The approval of ENCELTO marks a significant milestone in the treatment landscape for MacTel. MacTel has historically lacked effective treatment options, leaving patients with limited avenues to manage their condition. ENCELTO’s approval offers new hope, providing a targeted therapy that addresses the underlying disease mechanism rather than just managing symptoms. This breakthrough could potentially slow disease progression, improve visual function, and enhance the quality of life for MacTel patients.
Despite its promising entry, ENCELTO faces an increasingly competitive market as multiple biotech companies are advancing their own therapies for MacTel. Emerging treatments, including gene therapies, small molecules, and biologics, are gaining traction in clinical development. Companies exploring innovative delivery mechanisms, such as sustained-release implants or intravitreal injections with improved dosing schedules, may offer added convenience and improved outcomes. To maintain its competitive edge, ENCELTO’s developers will need to emphasize its unique clinical benefits, superior safety profile, and potential for long-term disease control.
Strategic positioning will be key for ENCELTO’s success. Building strong partnerships with retinal specialists, enhancing physician awareness through targeted education programs, and ensuring streamlined access to the therapy will be crucial. Additionally, securing favorable reimbursement strategies will play a pivotal role in expanding patient adoption, particularly in markets where treatment costs may present a barrier. By highlighting real-world evidence and long-term efficacy data, ENCELTO can strengthen its standing as a preferred option for both clinicians and patients.
Ultimately, while competition in the MacTel treatment landscape is poised to intensify, ENCELTO’s early market entry presents an advantage. By proactively addressing patient needs, enhancing stakeholder engagement, and investing in post-approval research to reinforce its benefits, ENCELTO can establish itself as a cornerstone treatment in this underserved therapeutic area.

Downloads
Article in PDF



