ENHERTU: Another Triumph to Celebrate for AstraZeneca and Daiichi Sankyo
Apr 15, 2024
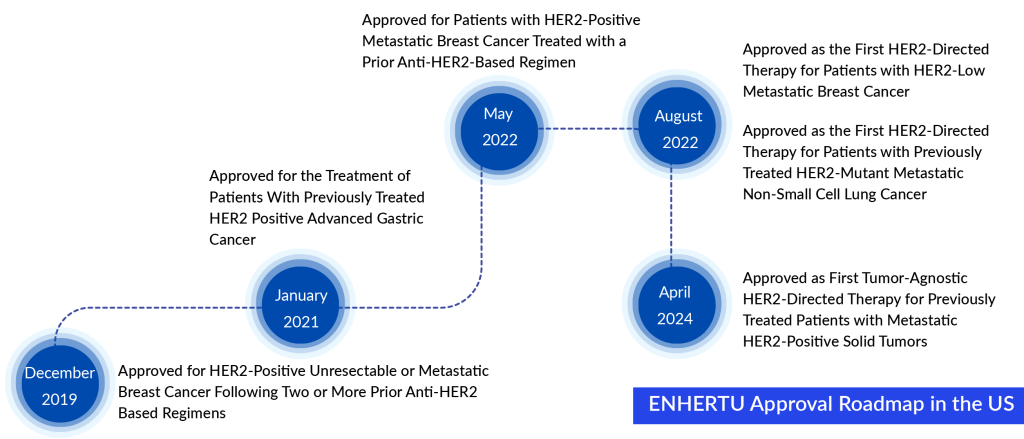
ENHERTU lies at the core of AstraZeneca and Daiichi Sankyo’s objectives for advancing in oncology. This collaboration has notably broadened the antibody-drug conjugates impact in the United States. On April 5, 2024, the FDA approved ENHERTU to treat HER2-positive solid tumors in adults who have received previous systemic treatment and have exhausted other available treatment options.
With this approval, ENHERTU achieves a groundbreaking milestone as the premier therapy targeting HER2 across various tumor types, marking the first ADC to secure such a broad indication. ENHERTU, an ADC targeting HER2, was developed by Daiichi Sankyo and is currently undergoing joint development and commercialization by both Daiichi Sankyo and AstraZeneca.
The first tumor agnostic approval was based on effectiveness findings from 192 adult patients with advanced HER2 positive (IHC 3+) solid tumors, previously treated and not suitable for surgery. These patients were part of three multicenter phase II trials within the DESTINY clinical development initiative—namely, DESTINY-PanTumor02, DESTINY-Lung01, or DESTINY-CRC02. Across all three studies, the primary measure of effectiveness was the confirmed Objective Response Rate (ORR), with another measure being the Duration of Response (DOR).
Downloads
Article in PDF
Recent Articles
- Bluebird suspends gene therapy trials; Cortexyme tripped up by FDA hold; Guardant Health launches...
- Why should every company working on CAR-T therapy attend CAR TCR Summit Europe?
- Another Feather in the Cap for Xtandi and Keytruda — The Two Main Cancer Drugs
- Eli Lilly’s Jaypirca Approval; Novartis’ Adakveo EMA Review; Janssen’s CARTITUDE-4 Study of CARVY...
- Edwards’ Sapien 3 with Alterra Prestent; Koios Medical’s breast, thyroid cancer-spotting AI; Line...
Dave Fredrickson, AstraZeneca’s Executive Vice President of the Oncology Business Unit, emphasized that ENHERTU, the initial antibody-drug conjugate with a tumor-agnostic indication, is effectively realizing its potential within metastatic HER2-targetable tumors. He also highlighted the significance of testing for biomarkers, such as HER2, in a wide array of tumors. This testing ensures that patients with advanced cancer, who have limited treatment choices, are informed about the possibility of a suitable targeted medication.
In the DESTINY-PanTumor02 trial, effectiveness was evaluated in a specific group of patients (n=111) who had received prior treatment, with HER2-positive (IHC 3+) solid tumors that were either centrally or locally confirmed, including biliary tract, bladder, cervical, endometrial, ovarian, pancreatic, or other types of tumors. The confirmed objective response rate (ORR) was 51.4% (95% confidence interval [CI]: 41.7-61.0), and the range for the median duration of response (DOR) was 19.4 months (with responses ongoing beyond 27.9 months [‘+’ indicates continuing responses at the data cutoff]).
In the DESTINY-Lung01 study, effectiveness was assessed in a subgroup of patients (n=17) with HER2-positive (IHC 3+) non-small cell lung cancer (NSCLC) that was centrally confirmed. The confirmed ORR was 52.9% (95% CI: 27.8-77.0), and the range for the median DOR was 6.9 months (with responses ongoing beyond 11.7 months [‘+’ indicates continuing responses at the data cutoff]).
In the DESTINY-CRC02 trial, effectiveness was evaluated in a subgroup of patients (n=64) with HER2-positive (IHC 3+) colorectal cancer that was centrally confirmed. The confirmed ORR was 46.9% (95% CI: 34.3-59.8), and the range for the median DOR was 5.5 months (with responses ongoing beyond 9.7 months [‘+’ indicates continuing responses at the data cutoff]).
The recent approval signifies ENHERTU’s fifth endorsed use in the United States. Initially authorized by the FDA in December 2019, ENHERTU was approved for treating HER2-positive unresectable or metastatic breast cancer in patients who have undergone two or more anti-HER2 therapies. Over time, ENHERTU has gained approval for various other uses, such as gastric cancer and non-small cell lung cancer.
Ken Keller, who serves as the Global Head of Oncology Business and as President and CEO of Daiichi Sankyo, Inc., expressed that gaining approval for ENHERTU as the fifth indication in the US marks a notable achievement. Patients who qualify, having previously received treatment for metastatic HER2-positive solid tumors, can now benefit from ENHERTU. The FDA’s accelerated approval for this broad indication, regardless of tumor type, reflects the significant effectiveness of ENHERTU observed across various forms of metastatic cancers.
The approval was granted after the FDA assessed the application through the Real-Time Oncology Review (RTOR) program, under Priority Review and Breakthrough Therapy Designation. This submission was examined within Project Orbis, a system facilitating simultaneous submission and review of cancer medications among participating global partners. Additionally, ENHERTU is currently undergoing regulatory assessment for the same purpose by authorities in Australia, Brazil, and Singapore as part of Project Orbis.
According to these findings, ENHERTU has been added to the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) as a viable treatment choice for various metastatic cancers.
The ADC comes with a boxed warning concerning interstitial lung disease and potential harm to embryos or fetuses. Those using ENHERTU should be closely monitored for indications like coughing, difficulty breathing, fever, and any other respiratory issues.
ENHERTU has emerged as a crucial asset in AstraZeneca and Daiichi Sankyo’s lineup. Throughout 2023, AstraZeneca’s revenue from ENHERTU reached $1.28 billion, comprising mostly profits and royalties from specific markets such as the US. The total sales of ENHERTU between the two companies reached $2.57 billion in 2023, more than doubling from its $1.25 billion earnings in 2022.
While AstraZeneca and Daiichi officials had expressed earlier concerns about their ADC’s growth plateauing in the HER2-low setting, the recent approval for use across various tumor types could potentially expand ENHERTU’s reach to ‘several thousands’ of patients. In January, Daiichi revised its overall sales forecast for ENHERTU upwards but simultaneously lowered the projection for the US market.
At that time, Daiichi anticipated ENHERTU sales to amount to 383.9 billion Japanese yen (approximately $2.6 billion) for the 12 months ending March 31, which included profits from regions where AstraZeneca manages ADC sales. Despite the increased global forecast, Daiichi reduced its 12-month sales estimate for the US by $30 million to $1.58 billion.
In summary, ENHERTU’s future outlook appears brilliantly promising following its recent FDA approval. With this green light from the FDA, ENHERTU is poised to redefine treatment standards, offering renewed hope to patients with previously limited options. The approval not only underscores its potential to significantly improve patient outcomes but also hints at its potential expansion into other HER2-expressing cancers, paving the way for a transformative impact on oncology treatment landscapes worldwide.
As ENHERTU strides into the market, its trajectory seems to align with a new era of precision medicine, where targeted therapies revolutionize how we approach cancer care. The FDA’s approval underscores not just the drug’s effectiveness but also its safety profile, heralding a new beacon of hope for patients and oncologists alike. Investors and analysts are eyeing ENHERTU with optimism, foreseeing a blockbuster potential that could reshape not just treatment protocols but also the future narrative of HER2-positive cancers. With ongoing research and potential label expansions, ENHERTU’s recent FDA approval is a pivotal moment, setting the stage for a brighter, more effective tomorrow in the fight against breast cancer and beyond.

Downloads
Article in PDF
Recent Articles
- Sanofi’s Rare Disease Drug Xenpozyme’ Approval; FDA Approves Novartis’ Pluvicto; AstraZenec...
- Copiktra receives approval; Lilly wins approval; Eisai’s Fycompa; Astellas’ Roxadustat; Janssen’s...
- Antibody-drug Conjugates in Oncology: An Overview of the Current and Future Treatment Landscape
- Gilead’s Livdelzi FDA Approval for Primary Biliary Cholangitis; Incyte and Syndax’s Niktimv...
- Imfinzi fails. What’s next in Non-small cell lung cancers Market Scenario?



