The Evolving Landscape of COPD Treatments: New Hope for Patients
Dec 20, 2024
Table of Contents
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death globally, according to the World Health Organization (WHO). It affects approximately 251 million people worldwide. Smoking is the leading cause of COPD, responsible for around 70-90% of cases. However, exposure to air pollution, occupational dust and chemicals, and genetic factors (such as alpha-1 antitrypsin deficiency) also contribute to the development of COPD.
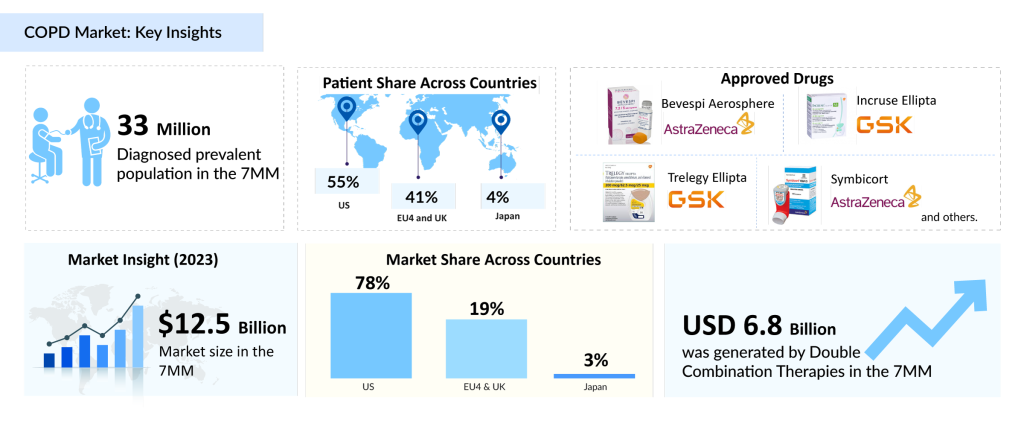
The diagnosed prevalent population of COPD in the 7MM was around 44 million in 2023. These cases of COPD in the 7MM are expected to increase at a significant CAGR of 1.4% throughout the study period (2020–2034), as per DelveInsight’s latest assessment in the Chronic Obstructive Pulmonary Disease Epidem Forecast Report.
Downloads
Article in PDF
Recent Articles
- Understanding the Chronic Obstructive Pulmonary Disease Increasing Prevalence and Trends
- Assessing the Growing Role & the Demand of Apps in Managing the Chronic Diseases
- DUPIXENT Receives First-Ever Biologic Approval for COPD: Adds Another Jewel in its Crown
- WORLD CHRONIC OBSTRUCTIVE PULMONARY DISEASE DAY
- Aktis’s Novel Targeted Alpha Radiopharmaceuticals; Koye Partners with Sonde Health; Novartis to S...
As per the estimates, the majority of cases of COPD are in females as compared to males, in the US. But in EU4 and the UK, and Japan the diagnosed cases of males represent the majority of the cases. Overall, in the 7MM, females are predominantly high in number.
In 2023, the US had around 3 million, 9 million, 5 million, and 1 million cases of GOLD 1, GOLD 2, GOLD 3, and GOLD 4, respectively based on the severity of airflow limitation. Estimates suggest that airflow limitation was higher in GOLD 2 severity.
Get more detailed insights about COPD at Understanding the Chronic Obstructive Pulmonary Disease Increasing Prevalence and Trends
How is COPD Currently Managed?
Various medications are used to manage the symptoms and complications of COPD, including bronchodilators, which help relax the muscles around the airways, making it easier to breathe by opening the airways. Most bronchodilators are delivered through inhalers, and in more severe cases, inhalers may also contain steroids to reduce inflammation.
Common short-acting and long-acting bronchodilators for COPD include Albuterol (ProAir HFA, Ventolin HFA, and others), Ipratropium (Atrovent HFA), Levalbuterol (Xopenex), Aclidinium (Tudorza Pressair), Arformoterol (Brovana), and Formoterol (Perforomist).
In the LABA category, medications such as Striverdi Respimat, Arcapta/Onbrez, Serevent, and Brovana are included, while the LAMA class features drugs like Spiriva (Spiriva HandiHaler and Spiriva Respimat), Tudorza Pressair, Incruse Ellipta, Yupelri, Seebri Neohaler, and Lonhala Magnair. Since the generic version of Brovana was approved in April 2020, its market share has decreased. Additionally, Arcapta Neohaler, Seebri Neohaler, and Utibron Neohaler were discontinued in the US in March 2020, no longer contributing to the market.
For patients who do not respond to single therapies, double or triple therapies are often prescribed. Among the double therapies, the LABA+ICS combination is commonly recommended, including drugs such as Symbicort, Breo Ellipta, and Advair. LABA+LAMA combinations, such as Anoro Ellipta, Stiolto Respimat, and Bevespi Aerosphere, are also available.
In September 2024, Sanofi/Regeneron Pharmaceuticals’ DUPIXENT achieved another milestone by receiving approval for label expansion in COPD from the FDA, EU, and NMPA. This approval makes DUPIXENT the first targeted therapy for COPD, marking a major breakthrough in the treatment of the disease. Unlike traditional treatments that mainly focus on symptom management, DUPIXENT targets the IL-4 and IL-13 pathways associated with type 2 inflammation, a key factor in certain COPD patients. This precise targeting enables more personalized treatment, particularly for patients with elevated eosinophils, who often face frequent exacerbations and poorer outcomes.
The Phase III BOREAS trial showed that patients receiving DUPIXENT had a 30% reduction in moderate-to-severe exacerbations over 52 weeks, along with significant improvements in lung function and quality of life. By Week 52, those on DUPIXENT had a 160 mL improvement in prebronchodilator FEV1, compared to 77 mL in the placebo group. DUPIXENT was also well-tolerated, with a low incidence of adverse events. These findings suggest that DUPIXENT could significantly change COPD management by reducing exacerbations, improving lung function, and providing a safer, more targeted treatment option for patients with type 2 inflammation.
Track the complete approval journey of DUPIXENT for COPD at “DUPIXENT Receives First-Ever Biologic Approval for COPD: Adds Another Jewel in its Crown”
In addition to these approved COPD drugs, several promising therapeutic options by leading companies are currently in development.
Emerging Therapies for COPD Treatment to Watch
Over 70+ drugs are currently in the pipeline across the globe, as per DelveInsight’s COPD Pipeline Insight Report. The chronic obstructive pulmonary disease pipeline possesses potential drugs in mid-stage developments to be launched in the near future. Itepekimab (Sanofi/Regeneron Pharmaceuticals), Benralizumab (AstraZeneca), Tezepelumab (Amgen/AstraZeneca), Mepolizumab (GSK), and others shall further create a positive impact on the COPD treatment market.
Itepekimab (SAR440340/REGN3500/Anti-IL-33 mAb): Sanofi/Regeneron Pharmaceuticals
Itepekimab is a fully human monoclonal antibody that targets interleukin-33 (IL-33), a protein implicated in type 1 and type 2 inflammation. Administered via subcutaneous injection, preclinical studies demonstrated that REGN3500 inhibited various markers of both types of inflammation. Regeneron and Sanofi are currently investigating REGN3500 in diseases involving inflammation, including respiratory and dermatological conditions. REGN3500 is undergoing Phase III trials for COPD. Developed using Regeneron’s VelocImmune technology, which optimizes fully human antibodies, it is being jointly developed by Regeneron and Sanofi under a global collaboration agreement.
FASENRA (Benralizumab): AstraZeneca
FASENRA (benralizumab) is a humanized recombinant monoclonal antibody of the IgG1k immunoglobulin class that specifically targets the alpha chain of the interleukin 5 receptor (IL-5R) found on eosinophils and basophils. By binding to the receptor, it prevents IL-5 from attaching and inhibits the hetero-oligomerization of the alpha and beta subunits of the IL-5R, thereby blocking signal transduction. Additionally, as an afucosylated IgG, it has a strong affinity for the FcγRIIIα receptor present in natural killer cells, macrophages, and neutrophils. Developed by MedImmune, AstraZeneca’s global biologics research and development division, benralizumab was approved by the FDA in November 2017 for severe eosinophilic asthma. The company is now advancing FASENRA in Phase III clinical trials for the treatment of COPD.

Mepolizumab: GSK
Mepolizumab is currently undergoing Phase III clinical trials for COPD, particularly in patients with frequent exacerbations and elevated eosinophil levels. It is available as an injectable solution and powder for solution for subcutaneous administration. The IL-5 receptor complex activates several signaling pathways, leading to the release of cytokines, neuromediators, chemokines, and kinases that drive eosinophil differentiation, proliferation, recruitment, and degranulation. Mepolizumab works by inhibiting the formation of the IL-5 receptor complex, thereby preventing eosinophil activation. This disruption impairs normal eosinophil maturation and function, reducing eosinophilic airway inflammation and promoting decreased eosinophil survival.
Tezepelumab: Amgen/AstraZeneca
TEZSPIRE (tezepelumab), a pioneering human monoclonal antibody developed by Amgen and AstraZeneca, has shown considerable therapeutic promise for several respiratory conditions. Originally developed for COPD, TEZSPIRE has demonstrated encouraging outcomes in decreasing moderate-to-severe exacerbations in patients with high blood eosinophil counts. Recent data from the Phase IIa COURSE Trial, shared at the ATS 2024 conference, emphasized TEZSPIRE’s ability to reduce COPD exacerbations in patients with different eosinophil levels.
Key Highlights from ATS 2024
As the 2024 American Thoracic Society (ATS) conference approached, anticipation mounted within the respiratory research community for insights into the latest advancements in the management and treatment of COPD. Among the prominent attendees who were slated to present their contributions to COPD research and development were key industry players such as Sanofi, Roche, GSK, Verona Pharma, Amgen, and others. Their presence underscored the collective dedication to addressing the multifaceted challenges posed by COPD and offered a glimpse into the cutting-edge therapies and innovations shaping the future of respiratory medicine.
Sanofi’s Presentations at ATS 2024: Key Insights
At ATS 2024, Sanofi presented significant data regarding DUPIXENT (dupilumab), their groundbreaking therapy for immune-mediated respiratory diseases. Key insights from two landmark Phase III studies in COPD were highlighted, demonstrating DUPIXENT’s potential as a transformative treatment option. Late-breaking results from the NOTUS and BOREAS studies provided further evidence of DUPIXENT’s efficacy, particularly in select COPD patients with uncontrolled symptoms and type 2 inflammation. Additionally, new findings from the BOREAS study shed light on biomarkers’ predictive role in DUPIXENT treatment response.
Overall, Sanofi’s presentations at ATS 2024 emphasized their commitment to advancing DUPIXENT as a pioneering therapy in immune-mediated respiratory diseases. In September 2024, the US FDA approved DUPIXENT (dupilumab) as an add-on maintenance treatment for adults with inadequately controlled COPD and an eosinophilic phenotype.
Verona Pharma’s Presentations at ATS 2024: Key Insights
At ATS 2024, Verona Pharma presented additional analyses from its Phase III ENHANCE studies on ensifentrine for COPD management. Key insights included a pooled analysis that demonstrated reductions in exacerbation rates, the potential of ensifentrine as a first-in-class dual inhibitor of PDE3 and PDE4 enzymes, and promising efficacy and safety endpoints. These findings highlighted ensifentrine’s potential as a significant therapy for COPD patients, with notable benefits in exacerbation reduction and symptom improvement. The presentations also delved into ensifentrine’s unique mechanism of action, offering a novel therapeutic approach for COPD. Overall, Verona Pharma’s presence at ATS 2024 underscored its commitment to advancing innovative treatments for respiratory diseases.
Roche’s Presentation at ATS 2024: Key Insights
Roche presented a systematic review of the safety profile of astegolimab, an anti-ST2 monoclonal antibody, in various clinical trials, including those for asthma, atopic dermatitis, COPD, and COVID-19 pneumonia. The review encompassed data from completed, randomized, double-blind, placebo-controlled trials involving over 900 patients. Astegolimab demonstrated favorable safety outcomes, with no significant safety concerns identified across the diverse patient populations studied. The presentation underscored the potential of astegolimab as a promising therapeutic option for inflammatory conditions, supported by its well-tolerated safety profile.
Amgen’s Presentation at ATS 2024: Key Insights
Amgen presented Phase IIa data from the COURSE trial for TEZSPIRE (tezepelumab-ekko) in COPD at ATS 2024. The trial had investigated tezepelumab in patients with moderate to very severe COPD, regardless of eosinophil levels, inflammatory drivers, emphysema, chronic bronchitis, or smoking status. Tezepelumab demonstrated a numerical reduction in the annualized rate of moderate or severe COPD exacerbations compared to placebo, with more significant reductions observed in patients with higher eosinophil counts. These promising results highlighted the potential of tezepelumab for addressing the significant unmet medical need in COPD treatment. Amgen actively planned Phase III development based on these findings.
Get an in-depth analysis of all the COPD abstracts presented at the ATS 2024 at COPD Analysis and Insights
COPD Treatment Space: A Bright Horizon Ahead
Chronic obstructive pulmonary disease has long been a challenging condition to manage, with millions worldwide grappling with its debilitating symptoms and progressive nature. However, the treatment landscape is witnessing transformative advancements, offering renewed hope to patients and healthcare providers alike. Novel therapies targeting the root causes of COPD, rather than just symptom management, are emerging as game-changers. The introduction of biologics, such as monoclonal antibodies targeting inflammatory pathways, is revolutionizing treatment paradigms by addressing underlying inflammation and disease progression. Coupled with advancements in inhalation therapies delivering ultra-long-acting bronchodilators and combination regimens, patients can now achieve better symptom control, reduced exacerbations, and improved quality of life.
DelveInsight estimates that the market size for COPD in the 7MM is expected to grow from USD 16 billion in 2023 with a CAGR of 5% by 2034. This growth is mainly driven by the rise in the number of prevalent cases of COPD, ongoing clinical research, and better diagnostic tools that might improve the prognosis of the disease.
Beyond pharmacological interventions, innovative digital health technologies are playing an integral role in COPD management. Remote monitoring tools, mobile health applications, and connected inhalers enable personalized care by tracking patient adherence, detecting early signs of exacerbations, and facilitating timely medical interventions. Furthermore, research into regenerative medicine, including stem cell therapies, holds the potential to repair damaged lung tissue, offering a curative approach to COPD. With an increasing focus on early diagnosis, multidisciplinary care, and equitable access to novel treatments, the COPD treatment space is evolving rapidly, presenting a bright horizon for the millions affected by this life-limiting disease.

Downloads
Article in PDF
Recent Articles
- Alarming Growth of Chronic Respiratory Diseases (CRDs) and Their Prolonged Impact on the Quality ...
- Can Dupixent Be A Gamechanger In The Atopic Dermatitis Treatment Landscape?
- Eisai Announces Solo Development of Farletuzumab Ecteribulin (FZEC); Johnson & Johnson’s Nipo...
- Aerin Medical’s RhinAer Stylus; CE Mark for Haemonetics’s VASCADE Vascular Closure Device; J&...
- Sanifit nets USD 80.9 M; FDA approves Dupixent for CRS; AbbVie buys Allergan