Advances in Prader-Willi Syndrome Treatment: New Hope for Patients
Apr 04, 2025
Table of Contents
Prader-Willi Syndrome is a complex genetic disorder characterized by a variety of symptoms, including hyperphagia, obesity, and behavioral issues. According to the Prader-Willi Syndrome Association USA, the disorder affects approximately 1 in every 15,000 live births, with more than 300,000 people impacted globally.
Based on DelveInsight’s assessment in 2023, the 7MM had approximately 25K diagnosed prevalent cases of PWS. These cases are expected to rise due to advancements in diagnostic capabilities, disease awareness, and the setting up of more PWS registries during the forecast period (2025−2034).
Current treatment options for PWS are limited, with management primarily focusing on lifestyle modifications to prevent obesity-related deaths. Nearly half of the deaths in PWS patients under 18 are linked to food-seeking behaviors such as choking and accidents.
The Prader-Willi syndrome treatment is tailored to address the specific symptoms present in each individual. Growth hormone replacement therapy is the only FDA-approved Prader-Willi syndrome treatment for children. Still, it is specifically approved to address growth failure and does not target hyperphagia or behavioral issues.
The FDA has approved three growth hormone products for PWS: GENOTROPIN, NORDITROPIN, and OMNITROPE. In contrast, both the EMA and MHLW have approved GENOTROPIN for PWS. Additionally, OMNITROPE is approved as a biosimilar for PWS in Europe.
The FDA has highlighted the lack of clearly defined mechanisms of action for treatments and the inadequacy of direct or surrogate study endpoints. The absence of established biomarkers for PWS, which could serve as surrogate endpoints, continues to be a major obstacle to successful studies. Historically, these studies have relied heavily on subjective caregiver questionnaires.
Soleno’s VYKAT XR Approval Sets a New Standard in Prader-Willi Syndrome Treatment
Since the FDA approved recombinant human growth hormone for Prader-Willi syndrome in 2000, several companies have struggled to develop more advanced treatments for hyperphagia, the insatiable hunger condition associated with PWS.
However, on March 26, Soleno Therapeutics achieved a breakthrough with VYKAT XR (diazoxide choline), which became the first drug approved to treat hyperphagia. VYKAT XR is approved for individuals aged 4 years and older with PWS, which results in low muscle tone, short stature, and intellectual and developmental challenges.
This once-daily extended-release tablet acts as a potassium channel activator. These channels, found throughout the body, including in the pancreas, help regulate insulin secretion. Based on the average weight of patients in clinical trials, Soleno plans to price VYKAT XR at $466,200 per year. Formerly known as DCCR, VYKAT XR is expected to be available in the US starting in April 2025.
The approval of VYKAT XR was supported by data from a Phase III trial in which patients were observed during a 16-week withdrawal study after taking VYKAT XR for a median period of 3.3 years. Those who switched to a placebo during the study demonstrated a statistically significant worsening of hyperphagia compared to patients who continued the medication.
In addition, VYKAT XR has shown a consistent safety profile over four years of data collected in four open-label studies. The most common side effects—hypertrichosis, edema, hyperglycemia, and rash—occurred in approximately 10% of the patients.
Prader-Willi Syndrome: A Graveyard of Hyperphagia Treatment Trials
Several companies have faced setbacks in their attempts to develop treatments for hyperphagia associated with Prader-Willi syndrome. Alize Pharma had success in a 2016 Phase II study with AZP-531, a first-in-class unacylated ghrelin peptide analog, but was unable to move the treatment forward. In 2016, Zafgen demonstrated the efficacy of beloranib, a MetAP2 inhibitor, in a Phase III trial, but the treatment was ultimately derailed due to safety concerns. Saniona developed Tesomet, a combination of the monoamine reuptake inhibitor tesofensine and beta blocker metoprolol. Tesomet received Orphan Drug designation from the FDA in 2021, but its development was halted in 2022 due to funding limitations.
Promising Candidates in Pipeline for Prader-Willi Syndrome Treatment
The Prader-Willi syndrome pipeline possesses some drugs in mid and late-stage development to be approved soon. The emerging landscape holds a diverse range of therapeutic alternatives for treatment, including H3 Histamine receptor inverse agonists/antagonists (WAKIX [pitolisant]), Oxytocin receptor agonists (Carbetocin [LV-101]), Targeting extra-oral bitter taste receptors (TAS2R) (ARD-101), MCHR1 protein inhibitors (RGH-706), and PDE-10 inhibitor (PBF-999), among others.
Harmony Biosciences’ WAKIX (pitolisant) is the only therapy being evaluated for PWS patients with Excessive Daytime Sleepiness (EDS), which is roughly 50% of the total PWS patients. We expect WAKIX to benefit from a longer period to peak in the market along with a strong commercial uptake owing to the high unmet need in this patient segment with almost no competition and an already established efficacy in its approved indication, Narcolepsy.
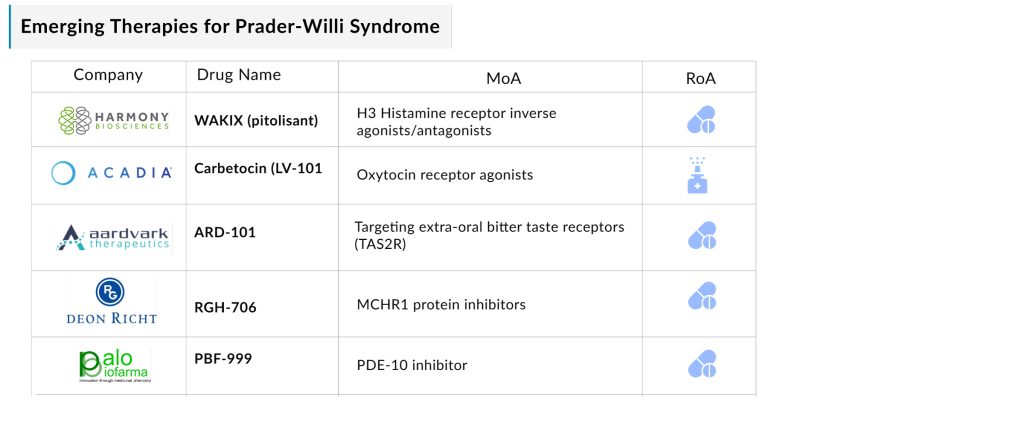
New companies are consistently entering the PWS treatment market. Key players currently include Harmony Biosciences, Soleno Therapeutics, ACADIA Pharmaceuticals, Aardvark Therapeutics, Gedeon Richter, Palobiofarma, Tonix Pharmaceuticals, and ConSynance Therapeutics, among others, who are working on developing therapies for PWS. The expected launch of therapies by these companies shall further create a positive impact on the PWS treatment market.
Key Developments in Prader-Willi Syndrome Treatment Space
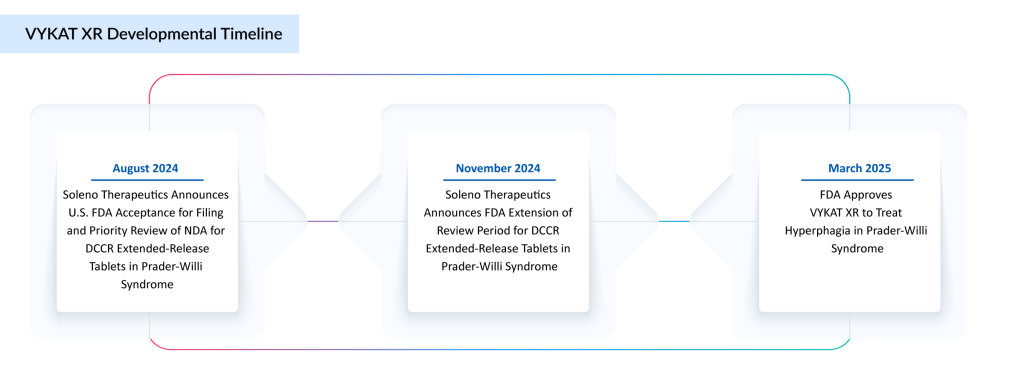
- In March 2025, Soleno Therapeutics announced that the FDA approved VYKAT XR (diazoxide choline) extended-release tablets for the treatment of hyperphagia in adults and children aged 4 years and older with Prader-Willi syndrome.
- In November 2024, Soleno Therapeutics announced that the FDA has extended the review period for its New Drug Application (NDA) for DCCR (diazoxide choline) extended-release tablets, intended to treat hyperphagia in Prader-Willi syndrome patients aged four and older.
- In August 2024, Soleno Therapeutics announced that the FDA had accepted its New Drug Application (NDA) for DCCR to treat Prader-Willi syndrome in individuals four years and older with hyperphagia.
- In July 2024, the FDA granted ConSynance Therapeutics a rare pediatric disease designation for its oral therapy aimed at treating Prader-Willi syndrome.
- In June 2024, Soleno Therapeutics submitted the NDA to the US FDA for approval of DCCR tablets for the treatment of PWS in individuals 4 years and older who have hyperphagia. The filing of the NDA with the US FDA follows positive data from an open-label extension Phase III clinical trial.
- In May 2024, Soleno Therapeutics announced the data from the randomized withdrawal period of Study C602 of DCCR tablets in PWS featured in an oral presentation at the Annual Meeting of the Endocrine Society (ENDO 2024).
- In March 2024, Pamplona announced that the FDA and the EMA granted orphan drug designation (ODD) to PBF-999 for the treatment of PWS.
- In February 2024, Harmony Biosciences announced that the US FDA had granted ODD to pitolisant for the treatment of PWS.
- In August 2023, Aardvark Therapeutics reported receipt of a rare pediatric disease designation in PWS from the FDA for its lead program, ARD-101.
Way Ahead in the Prader-Willi Syndrome Treatment
The future of Prader-Willi syndrome treatment is evolving beyond symptomatic management toward targeted therapies addressing the genetic and hormonal imbalances at the root of the disorder. The total market size of Prader-Willi syndrome in the 7MM in 2023 was approximately USD 650 million, this is anticipated to grow by 2034, driven by extensive market penetration of approved therapies in PWS due to label expansions and entry of new emerging therapies.
Advances in gene therapy, epigenetic modifications, and RNA-based treatments hold promise in correcting the underlying genetic defects associated with PWS, particularly the loss of function in the paternal chromosome 15 region. Researchers are also exploring the role of oxytocin and other neuropeptides in regulating appetite and social behavior, aiming to alleviate the hallmark hyperphagia and cognitive challenges of the syndrome. Additionally, improved formulations of growth hormone therapy and novel drug candidates targeting the hypothalamic dysfunction in PWS could offer more effective and personalized treatment options.
Beyond pharmacological approaches, multidisciplinary interventions, including AI-driven behavioral therapies, personalized nutritional plans, and digital health monitoring, are likely to enhance patient outcomes. The integration of wearable devices and remote patient monitoring could help caregivers and clinicians better track appetite, metabolism, and behavioral changes in real time, allowing for timely interventions. Moreover, ongoing PWS treatment clinical trials evaluating innovative approaches like gut microbiome modulation and deep brain stimulation may pave the way for more effective long-term management strategies. As research advances and regulatory approvals accelerate, a future with an improved quality of life and potential curative options for PWS patients seems increasingly attainable.




