Novartis’ FABHALTA: Leading the Way as the First Complement Inhibitor for IgAN
Aug 19, 2024
The first horse bearing Novartis’ IgAN treatment has reached the FDA’s approval milestone. FABHALTA is approved for use in treating IgAN patients who are at risk of swift disease progression, as evidenced by a urine protein-to-creatinine ratio of 1.5 g/g or higher.
On August 8, the FDA approved the use of Novartis’ complement inhibitor FABHALTA (iptacopan) to reduce proteinuria in patients with primary IgA nephropathy. This approval represents the first regulatory success for a complement inhibitor for this condition.
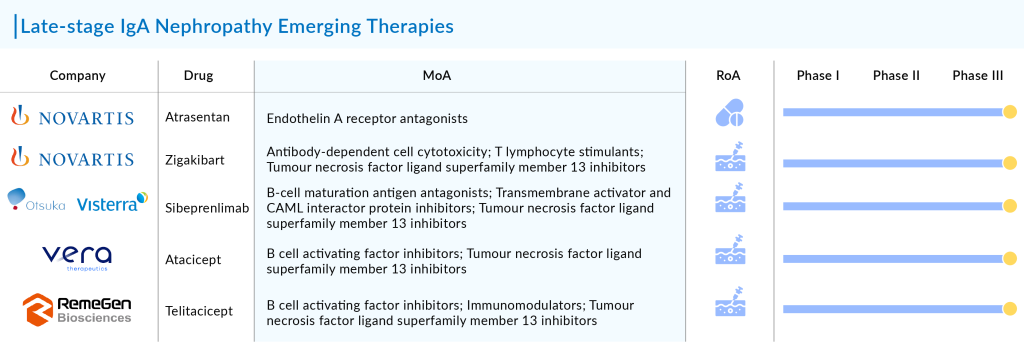
Novartis is developing three drugs for a rare autoimmune kidney disease: a complement factor B inhibitor, the endothelin A receptor antagonist atrasentan, and the anti-APRIL antibody zigakibart.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Novartis Announces the Positive Results of Phase III NATALEE Trial Evaluating Kisqali; FDA Approv...
- Diabetic Macular Edema Therapy Market to Witness Significant Impact in the Foreseeable Future
- FDA grants approval; Sanaria receives fast-track designation; Sanofi and Regeneron’s next blockbu...
- Snippet : Migraine
- Business Cocktail
FABHALTA, which received its initial FDA approval in December 2023 for the extremely rare blood disorder paroxysmal nocturnal hemoglobinuria, is now being considered for IgAN, a less rare but larger indication, approximately ten times the size of the PNH population. The total diagnosed prevalent population of IgAN in the 7MM comprised ~395K cases in 2023, as per DelveIsight.
Despite this, Novartis has maintained FABHALTA’s price at $550,000, which is significantly higher than the $120,000 wholesale cost of Travere Therapeutics’ competing drug, FILSPARI. Novartis also compared the price of FABHALTA to other complement inhibitors currently available for different diseases. For instance, AstraZeneca’s C5 inhibitor ULTOMIRIS, an infused antibody drug, is priced at over $500,000 for several autoimmune conditions, including PNH. In contrast, FABHALTA is an oral medication.
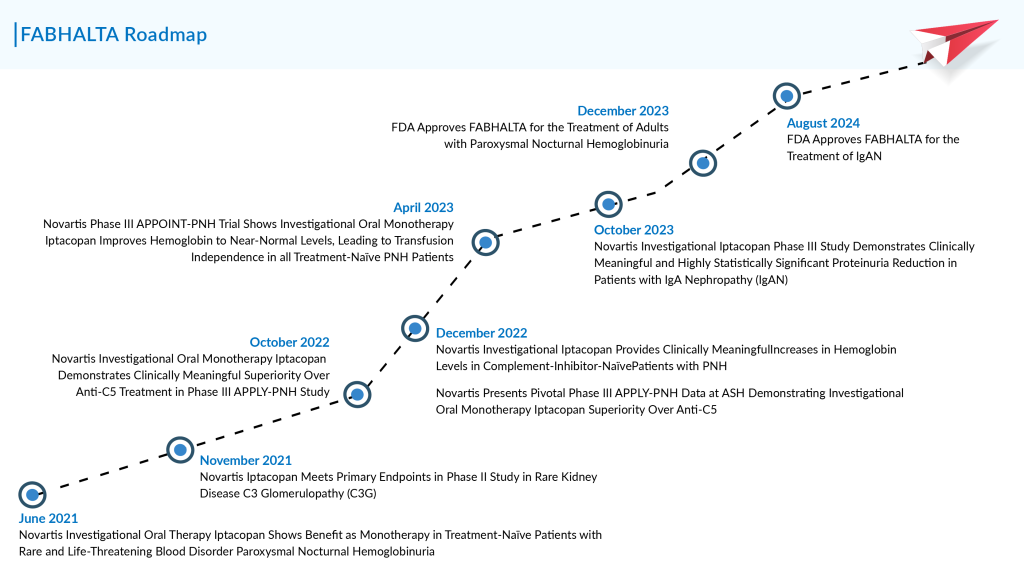
FABHALTA’s IgAN FDA approval was granted through the accelerated pathway, which requires Novartis to conduct a confirmatory Phase III study to verify the drug’s effectiveness for this use. The FDA’s decision was backed by data from the Phase III APPLAUSE-IgAN trial—a randomized, double-blind, parallel-group, placebo-controlled study with approximately 470 participants. In the trial, participants received 200 mg oral doses of FABHALTA twice daily, in addition to supportive care that included angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers.
In October 2023, Novartis announced that FABHALTA had successfully cleared the APPLAUSE-IgAN trial, though specific data were not initially provided. In a recent update, the company disclosed that FABHALTA reduced proteinuria by 44% over nine months, compared to a 9% reduction with placebo. Novartis highlighted that this reduction was both “clinically meaningful and statistically significant,” with a p-value under 0.0001.
The APPLAUSE-IgAN trial also confirmed FABHALTA’s safety for this use, with no new safety concerns. The most frequently reported side effects included respiratory tract infections, lipid disorders, and abdominal pain. FABHALTA’s label includes a boxed warning for serious infections related to encapsulated bacteria, such as Streptococcus pneumoniae and Neisseria meningitidis.
The APPLAUSE-IgAN study is still in progress and will keep assessing FABHALTA’s impact on slowing the deterioration of kidney function in patients, as measured by the estimated glomerular filtration rate. Novartis anticipates receiving results from this analysis in 2025, which will aid in obtaining traditional approval for FABHALTA.
FABHALTA is the first of Novartis’s three IgAN drugs to hit the market. However, atrasentan, which is currently under FDA review, is a closer competitor to Travere’s FILSPARI due to their similar mechanisms. FILSPARI acts as an endothelin type A (ETA) and angiotensin receptor inhibitor, while atrasentan, acquired by Novartis from its purchase of Chinook Therapeutics, is a selective ETA receptor antagonist.
Currently, a Phase III registration IgAN clinical trial (ALIGN) and a Phase II basket trial (AFFINITY) for primary glomerular diseases, including IgAN, FSGS, and Alport Syndrome, are evaluating the treatment.
At the 61st European Renal Association Congress, Novartis shared results from an interim analysis of the Phase III ALIGN study of atrasentan. Patients on atrasentan, in combination with supportive care (maintaining the highest tolerated dose of a renin-angiotensin system [RAS] inhibitor), saw a 36.1% reduction in proteinuria (measured by the 24-hour urine protein to creatinine ratio [UPCR]) at 36 weeks compared to those on placebo plus supportive care (p<0.0001). The study also confirmed that atrasentan has a safety profile consistent with previous data.
A reduction in proteinuria is a recognized marker for slowing the progression to kidney failure and has been used as an endpoint in IgAN clinical trials to support faster regulatory approvals. The FDA is expected to review atrasentan for IgAN in the first half of 2024.
The ALIGN study remains blinded, so only preliminary results are available. The final analysis, which will include changes in estimated glomerular filtration rate (eGFR) at 136 weeks and outcomes for participants receiving a sodium-glucose co-transporter-2 (SGLT2) inhibitor as background care in an exploratory cohort, is expected in 2026.
Despite their differing mechanisms, Novartis believes that each of its three drugs might be more suitable for patients with various disease presentations.
Before the approval of FILSPARI for IgAN treatment in early 2023, the only available treatments were steroids and off-label use of angiotensin-targeting ACE inhibitors and ARBs. Since FILSPARI operates through an ARB mechanism, patients currently on ARBs need to discontinue them before starting Travere’s therapy. Additionally, Novartis is likely to benefit from the fact that FABHALTA does not require routine liver function monitoring, unlike FILSPARI, which has a boxed warning for liver toxicity. However, FABHALTA comes with its own boxed warning regarding the risk of severe infections caused by encapsulated bacteria.
Travere Therapeutics’ FILSPARI is awaiting an FDA decision on full approval for IgAN treatment by September 5. Although the drug did not achieve statistical significance on its eGFR endpoint, Travere is hopeful that the consistent benefits observed across other metrics will persuade the FDA.
With the launch of FABHALTA for IgAN treatment, Novartis plans to collaborate with payers to secure coverage and is introducing a copay assistance program for those unable to afford it. Novartis will also focus on increasing awareness of the drug, as many patients and doctors prefer to avoid steroids due to their long-term side effects.
Besides PNH and IgAN, Novartis is exploring FABHALTA for several other rare conditions, such as C3 glomerulopathy, for which they aim to submit an FDA filing by the end of the year. The IgAN drug is also being studied for atypical hemolytic uremic syndrome, immune complex membranoproliferative glomerulonephritis, and lupus nephritis.
In addition to FABHALTA and atrasentan, Novartis is advancing another late-stage asset for IgAN: the subcutaneous anti-APRIL monoclonal antibody, zigakibart, which began Phase III development in July 2023.
Moreover, Novartis’ FABHALTA will also get fierce competition from other companies working with their late-stage assets. Key players in the development of IgAN therapies include Visterra (sibeprenlimab), Vera Therapeutics (Atacicept), and RemeGen (telitacicept), among others.
The expected launch of these IgAN therapies will transform the treatment landscape between 2024 and 2034. Until now, specific treatments for IgAN have been lacking, highlighting a crucial need for IgAN therapies that prevent progression to end-stage kidney disease (ESKD), which remains a primary unmet need. Major advancements in this field are likely to have a profound impact on the IgAN treatment market over the forecast period.

Downloads
Article in PDF
Recent Articles
- AbbVie Presents Phase III CANOVA Study Results; Novartis’ Iptacopan Shows Promise in Phase III St...
- Takeda knocks Velcade; FDA nod awaited; Novartis gets approval; Astellas UK dodges; Haegarda set ...
- COVID19 pipeline advances as Novartis begins phase III trial of hydroxychloroquine; and advanceme...
- Helsinn out licenses Pracinostat in South America
- Novartis’s Entresto; AZ, J&J and Lilly sharply cut death rates; CAR-T drugs worth; FDA warns