From NASH to MASH: Unraveling the Evolution and Future of Liver Disease Treatment
Oct 18, 2024
Table of Contents
In the ever-evolving world of liver disease, the shift from Nonalcoholic Steatohepatitis (NASH) to Metabolic Associated Steatotic Hepatitis (MASH) marks a revolutionary leap in our understanding and approach to treatment. This transition isn’t merely a rebranding; it signifies a profound recognition of the intricate dance between metabolic dysfunction and liver health. As obesity and metabolic disorders increasingly take center stage in public health, MASH emerges as a clarion call for a deeper dive into the metabolic roots of liver disease. Understanding the transition from NASH to MASH, alongside the evolving drug therapies, is vital for healthcare providers and patients navigating these complex conditions.
Decoding the NASH to MASH Transition
Nonalcoholic Steatohepatitis is becoming a highly epidemic condition, driven by rising obesity and type 2 diabetes rates. Left unchecked, NASH can lead to severe complications, including cirrhosis and liver failure, underscoring the urgent need for early intervention and treatment.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- CTEXLI Approved for Cerebrotendinous Xanthomatosis; SIGX1094 Wins Fast Track for Diffuse Gastric ...
- Tandem’s Automated Insulin Patch Pump; ResMed’s Bilevel Sleep Respiratory Devices; Masimo’s First...
- COMMERCIAL AND OTHERS
- Merck’s KEYTRUDA as Adjuvant Therapy for RCC Patients; BMS Receives Positive CHMP Opinion for CAR...
- FDA is reviewing a dangerous way to treat Peanut Allergy
MASH, formerly known as nonalcoholic fatty liver disease (NAFLD), which is now metabolic dysfunction-associated steatotic liver disease (MASLD), expands the scope of liver disease to encompass a wider range of conditions caused by metabolic dysfunction. This new classification acknowledges the intricate relationship between metabolic health and liver disease, reflecting the latest understanding of how fat accumulation in the liver can lead to progressive damage. In the face of these challenges, the pharmaceutical industry is at the forefront of developing innovative treatments aimed at halting or reversing the progression of liver diseases like NASH and MASH. As the global prevalence of these conditions continues to rise, driven largely by obesity and metabolic syndrome, the demand for effective therapies has never been more critical.
The future of liver disease treatment is one of hope and innovation, as the pharmaceutical industry continues to push the boundaries of what is possible in the fight against NASH and MASH. With a growing pipeline of promising therapies and a deeper understanding of the metabolic underpinnings of these diseases, we stand on the cusp of significant advancements that could transform the lives of millions affected by these conditions.
MASH Treatment Landscape: Patient Pool and Market Outlook
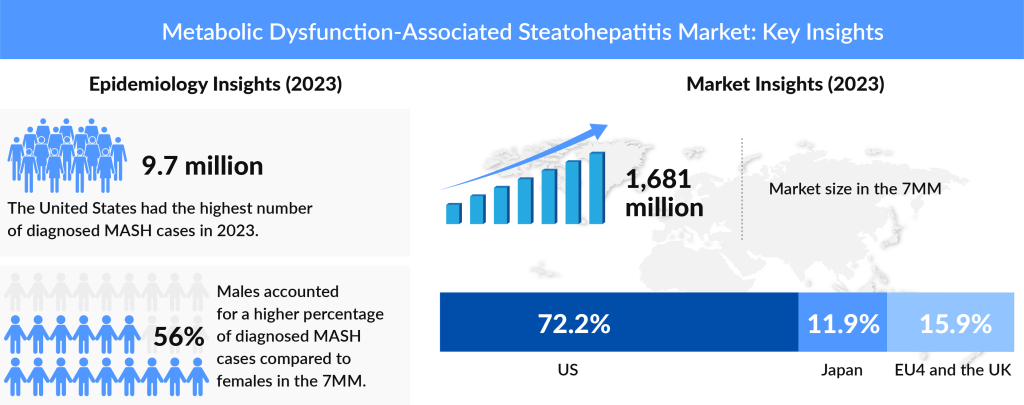
According to DelveInsight’s evaluation in 2023, there were an estimated 43 million prevalent cases of MASH in the 7MM. Out of these, a total of ~16 million cases were diagnosed, and this number is projected to increase by the end of 2034 in the 7MM.
In the US, it is estimated that 50% of individuals have been diagnosed with MASH, and this number is expected to increase during the forecast period. The rise in diagnosis rates can be attributed to improved screening processes, heightened awareness among healthcare providers, and the growing prevalence of risk factors such as obesity and metabolic syndrome.
Currently, only one drug is approved for MASH treatment. The approval of Madrigal Pharmaceuticals’ REZDIFFRA (resmetirom) by the US FDA in March 2024 marked a pivotal moment in the MASH treatment landscape. As the first FDA-approved therapy for non-cirrhotic MASH with moderate to advanced fibrosis (F2–F3), REZDIFFRA has set a new benchmark in MASH therapies.
Want the full scoop on REZDIFFRA’s rise? Check out our blog @ REZDIFFRA’s Trailblazing Journey in NASH Treatment
Additionally, the anticipated launch of Pegozafermin in the US market is expected to reduce the disease burden further, signaling a promising future for the MASH pipeline. As per DelveInsight analysis, the total market size of MASH in the 7MM was USD 1.7 billion. It is expected to grow at a significant CAGR, reaching a notable value by the end of 2034.
DelveInsight’s analysis forecasts market growth due to the introduction of emerging therapies, expecting a rise in market size during the study period (2020–2034). The anticipated increase in market size is driven by advancements in treatment options, greater healthcare access, and a rising prevalence of the condition, which together foster a higher demand for innovative and effective therapies.
Current Drugs in the Pipeline for NASH and MASH
Emerging therapies, particularly novel agonists, are game changers in the treatment of MASH. These innovative treatments target specific pathways involved in liver inflammation and fibrosis, offering new hope for the effective management of the disease. For instance, THR-β agonists like Madrigal Pharmaceuticals’ REZDIFFRA have shown promising results in reducing liver fat and inflammation, significantly advancing NASH therapy. The development of these emerging MASH drugs addresses unmet needs and enhances the potential for personalized treatment strategies, transforming the landscape of MASH care and improving patient outcomes.
- Semaglutide (Novo Nordisk): Originally approved for type 2 diabetes and obesity, semaglutide has shown significant improvements in liver fat content and fibrosis in MASH patients during Phase II trials. It is currently undergoing Phase III development for MASH.
- Lanifibranor (Inventiva Pharma): A pan-PPAR agonist that targets multiple pathways involved in MASH. Lanifibranor is in Phase III trials and has demonstrated positive results in improving liver histology and metabolic parameters.
- Obeticholic Acid (Intercept Pharmaceuticals): An FXR agonist that has shown improvements in liver histology in NASH patients. Although it received a complete response letter from the FDA, further studies are ongoing to address safety concerns.
- Efruxifermin (Akero Therapeutics): A novel Fc-FGF21 fusion protein that has shown significant reductions in liver fat and markers of fibrosis in early trials. It is currently in Phase IIb studies.
- Tirzepatide (Eli Lilly): A dual GIP and GLP-1 receptor agonist that has demonstrated promising results in reducing liver fat and improving metabolic parameters in NASH patients. It is currently in Phase III trials.
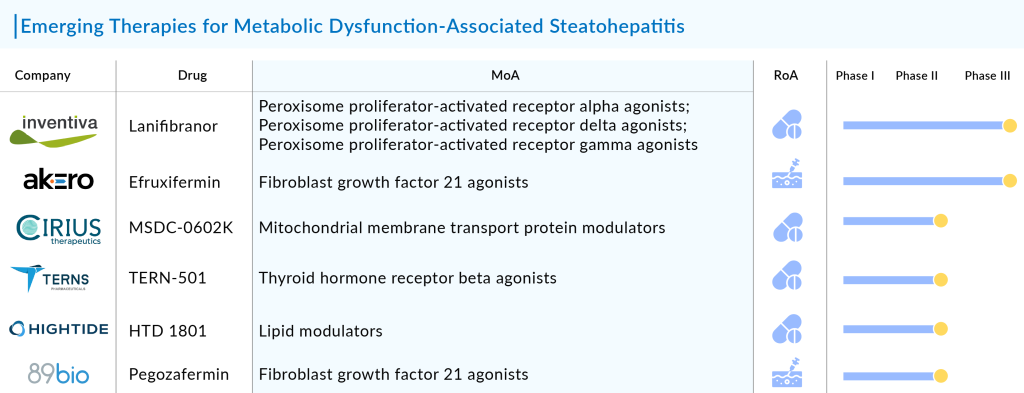
The other promising assests in the NASH pipeline include MSDC-0602K (Cirius Therapeutics, Inc.), Aramchol (Galmed Research and Development, Ltd.), Saroglitazar Magnesium (Zydus Therapeutics Inc.), HU6 (Rivus Pharmaceuticals, Inc.), EYP001 (Vonafexor) (Enyo Pharma), TERN-501 (Terns, Inc.), PXL065 (Poxel SA), HPG1860 (Hepagene (Shanghai) Co., Ltd.), Efinopegdutide (MK-6024) (Merck), Mitiperstat (AZD4831) (AstraZeneca), Denifanstat (formerly TVB-2640) (Sagimet Biosciences Inc.), Leronlimab (PRO 140) (CytoDyn, Inc.|Amarex Clinical Research), Icosabutate (NorthSea Therapeutics B.V.), Aldafermin (NGM282) (NGM Biopharmaceuticals, Inc), LPCN 1144 (Lipocine Inc.), HTD1801 (HighTide Biopharma Pty Ltd), Rencofilstat (Hepion Pharmaceuticals, Inc.), ALN-HSD (Alnylam Pharmaceuticals), GSK4532990 (GlaxoSmithKline), BI 456906 (Boehringer Ingelheim), and others.
Transitioning to MASH
In 2023, prominent liver disease organizations, including the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL), officially redefined Nonalcoholic Steatohepatitis (NASH) to Metabolic Dysfunction-Associated Steatohepatitis (MASH), aligning the name more closely with its metabolic roots. For the most up-to-date insights, explore our comprehensive MASH Market Report.
As the understanding of liver diseases evolves, the term MASH has emerged to more explicitly encompass the metabolic aspects of liver disease. This transition from NASH to MASH highlights an enhanced focus on metabolic dysfunction, acknowledging that liver disease is intricately linked with conditions such as obesity and type 2 diabetes. This shift acknowledges the significant role of metabolic dysfunction in liver conditions, particularly in patients with obesity and type 2 diabetes. The transition from NASH to MASH signifies a broader recognition of the underlying metabolic factors contributing to liver disease. To gain a deeper understanding of NASH, exploring its underlying mechanisms and the factors contributing to its progression is crucial.
Research into the disease’s pathophysiology is uncovering how fat accumulation in the liver leads to inflammation and fibrosis, which are central to NASH. By focusing on genetic and environmental risk factors, scientists aim to identify biomarkers that can predict disease progression and treatment responses. Additionally, integrating advances in precision medicine with emerging non-invasive diagnostic technologies promises to revolutionize our approach to managing NASH. This includes developing tools that can accurately assess liver health without the need for invasive procedures, thus allowing for earlier and more tailored interventions. As our knowledge of NASH evolves, these insights will help shape more effective strategies for both prevention and treatment.
Challenges and Opportunities in MASH Treatment
REZDIFFRA and other emerging therapies are at the forefront of innovation in treating MASH. REZDIFFRA, developed by Madrigal Pharmaceuticals, is a groundbreaking treatment for non-cirrhotic NASH with moderate to advanced liver scarring. As the first therapy specifically for this stage, REZDIFFRA offers new hope by targeting liver fat, inflammation, and fibrosis, marking a significant advancement in treating complex liver diseases.
Find out which late-stage NASH drugs will challenge REZDIFFRA’s supremacy
However, the journey is not without its challenges. The complexity of liver diseases such as NASH and MASH, coupled with varying patient responses to treatments, underscores the need for more extensive research and long-term safety data. The diverse nature of these conditions means that not all treatments will work equally well for every patient, and ongoing studies are crucial to understanding how to optimize treatment protocols and improve outcomes.
In conclusion, the journey from NASH to MASH illuminates a transformative era in liver disease treatment, where understanding and innovation are reshaping the landscape. As we navigate this evolving field, the shift to recognizing metabolic dysfunction’s role in liver conditions represents a significant leap forward. With pioneering therapies like REZDIFFRA and a growing pipeline of innovative treatments, the future of MASH management is brimming with potential. The fusion of advanced research, precision medicine, and non-invasive diagnostics heralds a new dawn in personalized care, offering hope and tangible improvements for millions affected by these chronic diseases. As we continue to unravel the complexities of liver disease, our commitment to addressing unmet needs and enhancing patient outcomes remains steadfast. The path from NASH to MASH is not just a progression in medical terminology but a beacon of progress and possibility in the fight against liver disease.

Downloads
Article in PDF