Gastroenteropancreatic Neuroendocrine Tumours Market To Gain Substantial Momentum With Entrance of Novel Therapies
Nov 01, 2024
Table of Contents
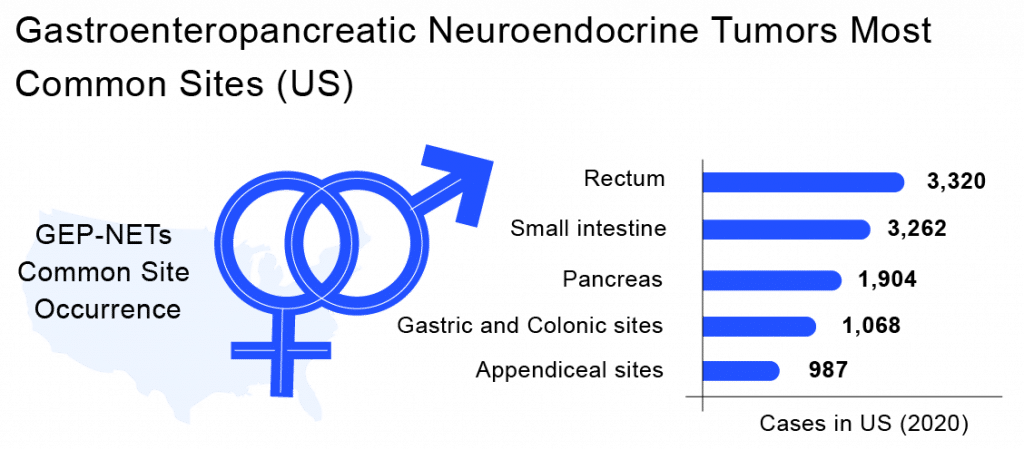
A Neuroendocrine tumor is a type of cancer where neuroendocrine cells develop into tumors. Whereas Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) are tumors that arise from neuroendocrine cells in the gut. A Neuroendocrine Tumor grows slowly and aggressively and tends to spread to other parts of the body. Many times neuroendocrine tumor symptoms aren’t detected incidentally. Because when GEP-NETs occur, their symptoms can vary on the basis of the location of the tumor. In rare cases, skin flushing or fluctuating blood sugar levels may occur. Gastroenteropancreatic Neuroendocrine Tumors symptoms depend on where the tumor is growing in the gut and whether it produces excess hormones. Gastroenteropancreatic Neuroendocrine Tumors can be life-threatening if they spread to other organs in the body. GEP-NETs give rise to a rare type of tumor that can form in the pancreas or in other parts of the gastrointestinal tract, including the stomach, colon, small intestine, appendix, and rectum. They are formed in the cells that secrete hormones. This extra hormone production is responsible for several signs and symptoms of disease, including a condition called carcinoid syndrome. GEP-NETs are tumors that arise from neuroendocrine may occur as either benign or malignant. Also, they are known as carcinoid tumors or islet cell tumors. By the time of GEP-NETs diagnosis, they might have spread to other organs as well. By morphology, GEP-NETs classification can be done by well-differentiated Neuroendocrine Tumors (NETs) or poorly differentiated neuroendocrine carcinomas (NECs). The main pathological variable determining Gastroenteropancreatic Neuroendocrine Tumors prognosis is tumor differentiation.
Gastroenteropancreatic Neuroendocrine Tumors Epidemiology
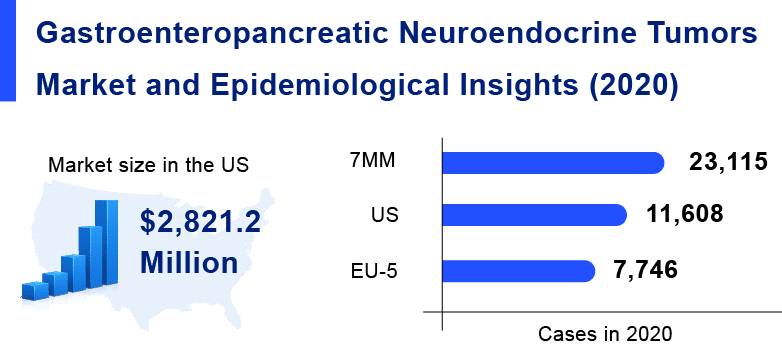
Gastroenteropancreatic Neuroendocrine Tumors are exclusively rare diseases, which might not affect a large population. As per Delveinsight analysis, in the 7MM countries [the United States, EU5 countries (Germany, France, Italy, Spain, and the United Kingdom), and Japan], the incident population of Gastroenteropancreatic Neuroendocrine Tumors was estimated to be almost 23k cases in 2020.
Downloads
Article in PDF
Recent Articles
- Evaluating the Present and Future Oncology Market Outlook With Valued Research and Analysis
- Most Promising Applications of Artificial Intelligence (AI) in Healthcare Segment
- Antibody-drug Conjugates in Oncology: An Overview of the Current and Future Treatment Landscape
- BGB-16673 Breakthrough: A Tolerable Triumph in Rapid Clinical Responses for Relapsed/Refractory B...
- Novel Insights Into The Non-Small Cell Lung Cancer Market
Gastroenteropancreatic Neuroendocrine Tumors are exclusively rare diseases, which might not affect a large population. As per Delveinsight analysis, in the 7MM, the incident population of Gastroenteropancreatic Neuroendocrine Tumors was estimated to be almost 23K cases in 2020.
Gastroenteropancreatic Neuroendocrine Tumors Treatment Scenario
Gastroenteropancreatic Neuroendocrine Tumors treatment depends on the type of tumor. Some of the approaches include surgery, radiation, and chemotherapy. Where surgery is considered to be the primary treatment option curative surgery should be considered even in the case of metastatic disease. For more progressed diseases, debulking and palliative surgery may reduce Gastroenteropancreatic Neuroendocrine Tumors symptoms load as well as facilitate and improve the outcomes from subsequent treatments. If present, hormonal symptoms can usually be ameliorated with somatostatin analogs (SSA), a second-line option is interferon-alpha (IFN-alpha). Other GEP-NETs treatments include molecular targeted therapy, chemotherapy, and peptide receptor radionuclide therapy (PRRT). For liver metastases, surgical resection, hepatic artery embolization (HAE), selective internal radiation therapy (SIRT), and radiofrequency ablation (RFA) or microwave ablation (MWA) can be performed.
Current GEP-NETs Marketed Therapies
Sunitinib maleate (marketed brand name – Sutent) is currently being manufactured and sold by the pharma giant – Pfizer. It is a tyrosine kinase inhibitor (TKI) that can irreversibly inhibit several kinases, including the VEGFR family, with anti-tumoral and antiangiogenic effects against several solid tumors. Pazopanib and axitinib are multi-targeted kinase inhibitors of VEGF receptors. They have shown some effect in patients with PNETs and advanced progressive extra-pancreatic Neuroendocrine Tumors
Peptide receptor radionuclide therapy (PRRT), either with 90-Yttrium-labeled compounds or with 177-Lu-DOTATATE (Lutathera), is internal radiotherapy that has been used for the past 15 years in uncontrolled trials in a variety of NET patients. In January 2018, FDA approved PRRT with the radiopharmaceutical – Lutetium 177 DOTA-TATE (Lu 177), for the treatment of somatostatin-receptor-positive Gastroenteropancreatic Neuroendocrine Tumors. Novartis produce PRRT with 177-Lu-DOTATATE and is currently the most widely used GEP-NETs therapy.
Somatuline Depot (marketed brand name – LANREOTIDE) is manufactured by Ipsen Pharmaceuticals. Somatuline is FDA-approved for the treatment of patients with unresectable, well- or moderately-differentiated, locally advanced, or metastatic and improves progression-free GEP-NETs survival.
Afinitor (marketed brand name – EVEROLIMUS) another drug produced by Novartis is a kinase inhibitor, indicated for the treatment of adult patients with progressive NET of pancreatic origin (PNET) and adults with progressive, well-differentiated, nonfunctional Gastroenteropancreatic Neuroendocrine Tumors.
Gastroenteropancreatic Neuroendocrine Tumors Pipeline Therapies & Emerging Drugs
There are several unmet needs in the Gastroenteropancreatic Neuroendocrine Tumors treatment landscape, such as a personalized treatment approach, challenges in diagnosis, epidemiology data collection, scarcity of guidelines, lack of specific and sensitive biomarkers, multidisciplinary approach, and finally, need for novel treatment options. Several companies are proactively developing new therapies to counter the current unmet needs of the Gastroenteropancreatic Neuroendocrine Tumors market and provide better treatment options. The current pipeline is extensive and consists of many key assets. The major players working in the Gastroenteropancreatic Neuroendocrine Tumors pipeline space are ITM Isotopen Technologien Muenchen, Camurus AB, Hutchison Medipharma Limited, Bristol-Myers Squibb, Eisai Limited, Experior S.L., Tarveda Therapeutics, Exelixis, Merck Sharp & Dohme Corp, Hoffmann-La Roche, Recordati Inc & Novartis Pharmaceuticals, Eli Lilly and Company, Trio Medicine, and many others.
GEP-NETs Market
As per Delveinsight’s analysis, the market size of gastrointestinal neuroendocrine tumors in the 7MM was USD 2.8 billion in 2020. Among the 7MM, the US accounted for 64% of the overall market size of GEP-NETs in 2020.
The GEP-NETs market is anticipated to reach heights in the coming years owing to the launch of upcoming novel therapies and the entrance of key pharmaceutical players in developing targeted therapeutics. These major GEP-NETs pharmaceutical players include names such as ITM Isotopen Technologien Muenchen, Camurus AB, Hutchison Medipharma Limited, and others whose key GEP-NETs therapies are expected to be launched in the market.
Gastroenteropancreatic Neuroendocrine Tumour (GEP-NETS) Market and Epidemiology Details
GEP-NETs Emerging Therapies
177Lu-edotreotide is an investigational ITM’s therapeutic radiopharmaceutical candidate that consists of two components: the medical radioisotope no-carrier-added lutetium-177 (n.c.a. 177Lu) and the targeting molecule edotreotide, a synthetic form of the peptide hormone somatostatin that targets NET-specific receptors. N.c.a. lutetium-177 is internalized into the tumor cells and decays, releasing medical radiation (ionizing beta-radiation) with a maximum radius of 1.7 mm and destroying the tumor. The highly precise localization can result in the healthy tissue surrounding the targeted tumor being minimally affected.
CAM2029 is an investigational pipeline drug by Camurus AB which is a ready-to-use, long-acting, subcutaneous depot of octreotide under development to treat three rare diseases; acromegaly, GEP-NET, and polycystic liver disease (PLD). Completed studies suggest that CAM2029 could offer significantly higher bioavailability and octreotide exposure, potentially enhancing treatment effectiveness compared to leading products on the market. Designed for ease of self-administration, CAM2029 includes a pre-filled pen option and utilizes Camurus’ proprietary FluidCrystal technology. Currently, CAM2029 is in Phase III clinical development for treating GEP-NET.
Surufatinib (previously known as HMPL-012 or sulfatinib) is currently being investigated by Hutchison Medipharma Limited. It is a novel, oral angio-Immuno kinase inhibitor that selectively inhibits the tyrosine kinase activity associated with vascular endothelial growth factor receptor (“VEGFR”) and fibroblast growth factor receptor (FGFR). FDA approved the New Drug Application (“NDA”) for Surufatinib for the treatment of pancreatic and extra-pancreatic (non-pancreatic) Neuroendocrine Tumors (NETs).
Nivolumab is an experimental novel therapy being studied by Bristol-Myers Squibb. It is a human immunoglobulin G4 (IgG4) monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD1 pathway-mediated inhibition of the immune response, including the antitumor immune response. The drug is currently being evaluated in combination with ipilimumab and temozolomide to treat NET of the pancreas or GI origin.
ITM-11 (177Lu-edotreotide), developed by ITM Isotope Technologies Munich, is an advanced Targeted Radionuclide Therapy composed of two key components: Edotreotide (DOTATOC), an octreotide-derived somatostatin analog, and EndolucinBeta, a synthetic, no-carrier-added (n.c.a.) lutetium-177 chloride, which is a low-energy beta-emitting radioisotope. ITM-11 is currently under investigation in two Phase III clinical trials, COMPETE (NCT03049189) and COMPOSE (NCT04919226). The COMPETE trial is assessing ITM-11 for grade 1 and grade 2 GEP-NETs, while the COMPOSE trial is focused on patients with well-differentiated high grade 2 and grade 3 GEP-NETs. Based on promising Phase II data that showed substantial improvement in progression-free survival (PFS), ITM-11 has also been granted orphan designation as a treatment for GEP-NETs.
Lenvatinib, discovered and developed by Eisai, is a kinase inhibitor that inhibits the kinase activities of vascular endothelial growth factor (VEGF) receptors VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4). The drug is currently being evaluated in Phase II of its clinical studies to assess the efficacy of lenvatinib in Metastatic Neuroendocrine Tumors.
PEN-221 is an experimental drug being investigated by Tarveda Therapeutics, it is a miniature drug conjugate designed to rapidly penetrate deep into solid tumors where it is highly selective for the somatostatin receptor 2 (SSTR2) and accumulates its potent DM1 payload. PEN-221 is being evaluated in Phase IIa expansion cohorts enrolling patients with mid-gut Neuroendocrine Tumors, Pancreatic Neuroendocrine Tumors, and small-cell lung cancer.

Cabozantinib is discovered and is currently being studied by Exelixis, it is a targeted agent that inhibits the activity of receptor tyrosine kinases, including MET, AXL, VEGF receptors, and RET. Exelixis discovered cabozantinib internally and maintains exclusive rights to commercialize the product in the United States. Outside of the United States, Ipsen has exclusive commercialization and development rights for current and future cabozantinib indications, except in Japan, where Exelixis has granted exclusive rights to Takeda. The drug is currently in preregistration phase.
KEYTRUDA is currently being investigated by Merck Sharp & Dohme Corp, which is a humanized monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including antitumor immune response. The drug is being evaluated as mono and combination therapy in Phase II and Phase I/II studies for the treatment of Neuroendocrine Tumors, Gastroenteropancreatic Neuroendocrine Tumors, and well-differentiated Neuroendocrine Tumors.
Entrectinib has been discovered and is presently being developed by Hoffmann-La Roche, it is an investigational CNS-active, oral small molecule inhibitor of pan-TrkA/B/C, ROS1, and ALK, which is designed to target tumors that harbor activating mutations to neurotrophic tropomyosin receptor kinase (NTRK) 1/2/3, the proto-oncogene ROS1, or anaplastic lymphoma kinase (ALK). The drug is currently being evaluated in Phase II of its clinical trials to treat patients with locally advanced or metastatic solid tumors that harbor NTRK1/2/3, ROS1, or ALK gene rearrangements.
Verzenio is an experimental novel therapy being studied by Eli Lilly and Company. It is a targeted treatment known as a CDK4/6 inhibitor, the drug is a non-chemotherapy oral tablet. It is currently being evaluated in Phase II of its clinical trials in patients with advanced and refractory well-differentiated GEP NETs. Ramucirumab is another investigational drug being studied by Eli Lilly and Company that works by blocking a receptor for a vascular growth factor, thereby preventing new blood vessels from forming. This may stop cancer from growing or spreading, and the tumor cells may die. The drug is being studied as a combination therapy. One Phase II trial is evaluating ramucirumab with somatostatin analog therapy in patients with advanced, progressive carcinoid tumors, while the other RamuNET trial aims to identify the effectiveness of ramucirumab with dacarbazine (DTIC) in progressively advanced PNET patients.
Belzutifan is an experimental drug being investigated by Merck Sharp & Dohme Corp which is a small molecule, that may help attack cancer cells by interfering with the binding of hypoxia-inducible factors HIF-2α and HIF-1B. It is currently being evaluated in Phase II of its clinical studies to evaluate the efficacy and safety of monotherapy in participants with advanced pheochromocytoma/paraganglioma (ppgl) or pancreatic NET (pNET). Regorafenib is another investigational drug studied by Merck, it is a kinase inhibitor used to treat patients with metastatic colorectal cancer, unresectable, locally advanced, or metastatic gastrointestinal stromal tumors, and hepatocellular carcinoma, in addition to Avelumab is a human anti-programmed death ligand-1 (PD-L1) antibody. The addition of regorafenib to avelumab has demonstrated modest antitumor responses and survival benefits in patients with cancer. The combination is currently being evaluated in a Phase II study in solid tumors.
Lurbinectedin (also known as PM01183, commercially Zepzelca) is an investigational therapy being studied by PharmaMaris an analog of a similar natural compound ET-736 with characteristics that make it similar but different to its parent compounds. The drug is being evaluated in combination with irinotecan to treat GEP-NET in Phase I/II.
Atezolizumab + Tivozanib is the combination being investigated by Genentech, Inc., and Aveo Oncology Pharmaceuticals in Phase I/II in immunologically cold tumor types.
Netazepide is an experimental therapy investigated by Trio Medicine. It is a potent, highly selective, insurmountable, and orally active gastrin blocker. The study was scheduled to start in the fourth quarter of 2020 and last three years.
Discover more about Top 5 Cancers Creating Major Challenge To The Global Healthcare System Here
Gastroenteropancreatic Neuroendocrine Tumors Future Update
Traditional Neuroendocrine Tumors treatments usually induce tumor stabilization for a limited length of time. Enormous effort is being put into developing novel approaches to overcome treatment-related resistance in patients with advanced and progressive Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs). The relatively small number of patients included in clinical studies, as well as the relatively slow course of the disease, make it difficult to evaluate the response rates for new therapies and their influence on GEP-NETs survival.
There are still many unmet needs in the therapeutic arsenal of GEP-NETs (e.g., the optimal sequencing of treatment modalities, exploration, and validation of different biomarkers, etc.). However, new insights into molecular alterations of Neuroendocrine Tumors should eventually improve the understanding of their complexity, facilitating a personalized approach and a successful Gastroenteropancreatic Neuroendocrine Tumors treatment for each patient in the future.

Downloads
Article in PDF
Recent Articles
- Data readout of potential ADCs: Enhertu gears up for Urothelial Carcinoma Market now
- Antibody-Drug Conjugates Market Outlook, 2015 Report
- DelveInsight’s Oncology based Gene Therapy Reports
- KaliVir, Astellas licensing deal; AbCellera’s IPO; Bayer CAR-T Cell therapy collab with Ata...
- GBT, Syros; Takeda, Cevance collaborates; IND application to ZN-c3