The Evolved Gene Therapy for Hemophilia
Nov 08, 2021
Hemophilia is an inherited rare disorder where blood doesn’t clot in the regular way because the person affected doesn’t make enough blood-clotting proteins (clotting factors). Without these factors, patients cannot stop bleeding when they are injured. Patients with more severe forms of the disease can experience spontaneous bleeding around the joints. Small cuts are not much dangerous but internal bleeding is very harmful to hemophiliac patients. The main concern is internal bleeding in elbows, knees, ankles and other joints. Internal bleeding can damage the organs and tissues and can also be potentially life-threatening.
Below mentioned are the different types of Hemophilia
- Hemophilia A: It is caused by a lack of blood clotting factor VIII; approximately 85% of Hemophiliacs have type A disease.
- Hemophilia B: It is caused by a deficiency of factor IX.
- Hemophilia C: Some doctors use this term to refer to a lack of clotting factor XI.
- Von Willebrand disease: A part of the factor VIII molecule known as von Willebrand factor or ristocetin cofactor is reduced. The von Willebrand factor involves helping the platelets (blood cells that control bleeding) attach to the lining of a vein or artery. This missing factor results in prolonged bleeding time because the platelets are unable to attach to the wall of the vessel and form a plug to stop the bleeding. This disease is similar to Hemophilia but is not usually called by this name. It is more common and usually milder than Hemophilia.
| Types of Hemophilia | Deficient Factor | Inheritance Type |
| Hemophilia A | VIII | X-linked recessive |
| Hemophilia B | IX | X-linked recessive |
| Hemophilia C | XI | Autosomal recessive |
| von Willebrand disease | VIII:vW | Autosomal dominant |
Hemophilia diagnosis includes screening tests such as Complete Blood Count (CBC), Activated Partial Thromboplastin Time (APTT) Test, Prothrombin Time (PT) Test, Fibrinogen Test and clotting factor tests. Screening tests are blood tests that show if the blood is clotting properly. Clotting factor tests, also called factor assays, are required to diagnose a bleeding disorder. This blood test shows the type of Hemophilia and the severity.
Downloads
Article in PDF
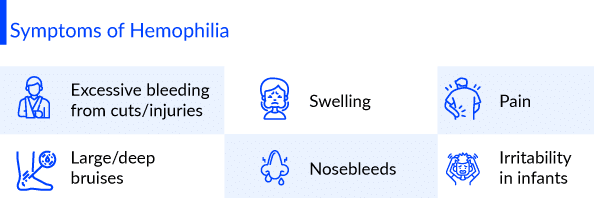
Hemophilia symptoms depend on the level of clotting factors. If the clotting-factor level is mildly reduced, it might bleed only after surgery or trauma. If the deficiency is severe, one can bleed easily for seemingly no reason. Hemophilia symptoms include unexplained and excessive bleeding from cuts or injuries, or after surgery or dental work, large and deep bruises, abnormal bleeding after vaccinations, pain, swelling, tightness in joints, blood in your urine or stool, nosebleeds and at times unexplained irritability in infants. Hemophilia risk factors include having a family member who also has the disorder. Males are much more likely to have Hemophilia than are females.
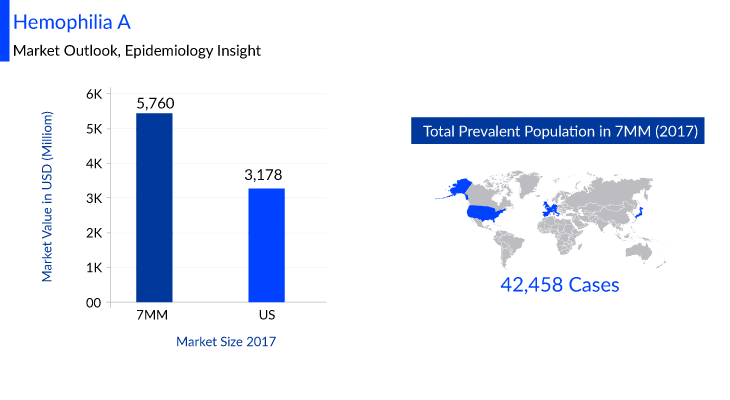
According to DelveInsight, the Hemophilia A market size in seven major markets (7MM) is estimated to be USD 5,760 million in 2017. Many key pharmaceuticals are proactively involved in producing Hemophilia A therapies including companies like Spark Therapeutics, Sigilon Therapeutics, ASC Therapeutics, Pfizer, Sanofi Genzyme, Novo Nordisk, and many others.
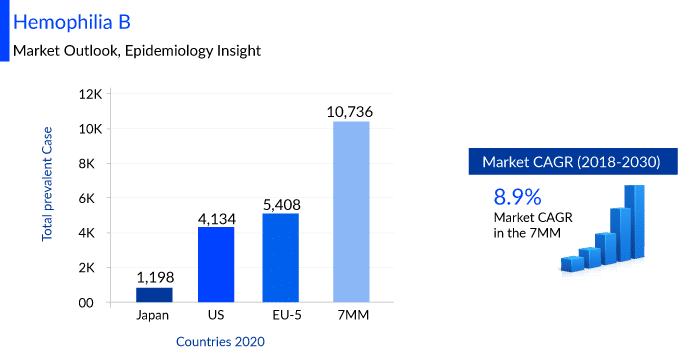
DelveInsight estimates that the Hemophilia B market size in the 7MM is expected to change at a CAGR of 8.9% by 2030 and the total number of prevalent cases of Hemophilia B was observed to be 10,739 cases in the 7MM in the year 2020. Many key pharmaceuticals are proactively involved in producing Hemophilia B therapies including companies like UniQure Biopharma B.V./CSL Behring, Sangamo Therapeutics, Pfizer, Spark Therapeutics, Genzyme, Novo Nordisk, Sanofi, ApcinteX Ltd, Freeline Therapeutics, and others.
As per DelveInsight, Hemophilia C occurs with an estimated prevalence of 1 case per 100,000 population in the United States, a rate that makes Hemophilia A approximately 10 times more common than Hemophilia C.
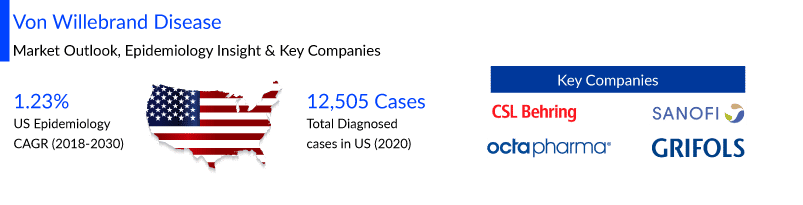
von Willebrand disease (VWD) affects up to 1% of the population in the United States, as analyzed by Delveinsight. von Willebrand disease market size in the US is anticipated to increase with a CAGR of 1.23% by 2030 and the total diagnosed von Willebrand disease prevalent cases were found to be 12,505 cases in the US in the year 2020. The leading companies working proactively to improve the VWD treatment landscape include CSL Behring GmbH, Sanofi-Aventis, Octapharma, Grifols, Takeda, and others.
Historically, there has been no cure for Hemophilia. Hemophilia treatment involves replacing the missing blood clotting factor so that the blood can clot properly, called replacement therapy. This is typically done by injecting treatment products, called clotting factor concentrates, into a person’s vein. Clinicians typically prescribe treatment products for episodic care or prophylactic care. Episodic care is used to stop a patient’s bleeding episodes; prophylactic care is used to prevent bleeding episodes from occurring.
Conceptually, clotting factors can help restore normal blood clotting in a patient, and this aspect can be achieved through gene therapy. Gene therapy for Hemophilia is touted as the most advanced and revolutionary treatment. A single dose of experimental gene therapy can boost the production of a missing blood-clotting factor in individuals with Hemophilia, giving patients a long-term solution for preventing dangerous bleeding episodes.
How can Gene Therapy be used in Hemophilia treatment
Gene therapy for Hemophilia involves inducing a modified virus (disease-free) in the hemophiliac patient to introduce a copy of the gene which encodes for the clotting factor that has been missing in patients. Following the gene therapy treatment with the virus, it is expected that the patients shall begin producing their own clotting factors. Human clinical trials have commenced in order to test potential gene therapies for Hemophilia.
CRISPR/Cas9 is another strategy that could allow a patient’s body to produce its own blood clotting factor. It uses a piece of genetic material and an enzyme that acts like molecular scissors to repair the genetic fault that causes clotting factor deficiency.
Gene therapy offers the potential for a cure for patients with Hemophilia by establishing continuous endogenous expression of factor VIII or factor IX (FIX) following the transfer of a functional gene to replace the hemophilic patient’s own defective gene.
Experimental Gene Therapies in Hemophilia
UniQure is developing two investigational gene therapies for Hemophilia AMT-060 and AMT-061. AMT-060 is an ongoing two-cohort Phase 1/2, non-randomized, open-label, multi-centre clinical trial (NCT02396342). There are 10 patients in the clinical study experiencing severe to moderately severe Hemophilia B. After administration of the AMT-060 gene therapy, clinical demonstration indicated a significant and sustained increase in factor IX activity, a substantial reduction in factor IX replacement therapy usage, and a complete cessation of spontaneous bleeding episodes. Now, AMT-061 is being developed to deliver a variant of the F9 gene that encodes for clotting factor IX called FIX-Padua. This variant carries the instructions to make factor IX protein that is eight times more active than the normal protein. A harmless virus vector is used to direct the delivery of FIX-Padua to the patient’s body. Interim results of an ongoing Phase 2B study (NCT03489291) testing the safety and efficacy of AMT-061 in three patients with severe to moderately severe Hemophilia B showed increased factor IX activity, reduced risk of bleeding, and no adverse events. A multicenter Phase 3 study (NCT03569891) evaluating the safety, efficacy, and tolerability of AMT-061 in Hemophilia B patients is also underway.
FLT180a is a gene therapy being developed by Freeline. A Phase 1 clinical trial (NCT03369444) is currently recruiting patients with Hemophilia B in the U.K. to test FLT180a. A second Phase 2/3 study (NCT03641703) is also recruiting participants in the same location to investigate the long-term safety and durability of factor IX activity in participants who have been treated with FLT180a gene therapy.
Sangamo Therapeutics is developing a genome editing therapy for Hemophilia B called SB-FIX. A Phase 1/2 clinical trial (NCT02695160) is currently recruiting participants at several sites in the U.S. and the U.K. SB-525 is another gene therapy for Hemophilia A developed by Sangamo Therapeutics. A Phase 1/2 clinical trial (NCT03061201) is currently recruiting about 20 adults with Hemophilia A to evaluate the safety, efficacy and tolerability of the treatment across many sites in the United States.

Fidanacogene elaparvovec (SPK-9001) is a gene therapy for Hemophilia B being developed in a partnership between Spark Therapeutics and Pfizer. This therapy is currently being investigated in a Phase II clinical trial (NCT02484092).
SPK-8011 is another gene therapy for Hemophilia A being developed by Spark Therapeutics. Preliminary results from Phase 1/2 clinical trials (NCT03003533) indicated that all five participants in the first two dose cohorts have shown persistent, stable clotting factor levels in their blood. Spark Therapeutics is developing another gene therapy called SPK-8016, which is designed to help Hemophilia A patients who have developed inhibitors against their own clotting factors. Patients are currently being recruited for a Phase 1/2 clinical trial (NCT03734588) in the U.S. to determine the effective dosage of the treatment.
Valrox (valoctocogene roxaparvovec or BMN 270) is a gene therapy for Hemophilia A being developed by Biomarin. The therapy is currently being tested in two Phase III clinical trials (NCT03392974 and NCT03370913).
| Company Name | Gene Therapy | Clinical Trial Phase |
| Biomarin | Valrox | Phase III |
| Spark Therapeutics and Pfizer | SPK-9001 | Phase II |
| UniQure | AMT-061AMT-060 | Phase IIPhase I/II |
| Spark Therapeutics | SPK-8011 SPK-8016 | Phase I/IIPhase I/II |
| Sangamo Therapeutics | SB-FIXSB-525 | Phase I/IIPhase I/II |
| Freeline | FLT180a | Phase I |
Gene Therapy for Hemophilia: Progress and Setbacks
Using gene therapy for Hemophilia treatment is in many ways a promising option, but it also has some drawbacks. Few of the progressive factors that gene therapy benefits in Hemophilia are:
- Hemophilia is considered to be an ideal genetic disorder to treat involving the gene therapy mechanism, as it occurs because of a single genetic defect i.e. a monogenic disease. This type of treatment can be considered immensely successful even if only a minimal increase in clotting factors is observed after the treatment. A little increase can lead to massive benefits for the patients.
- Another major advantage in gene therapy for Hemophilia is the way of disease occurrence in terms of a genetic disorder. It is very simple, caused by one known genetic defect that decreases the body’s ability to produce sufficient clotting factors in the blood. Unlike other genetic diseases, which at times involve numerous genetic defects, Hemophilia is comparatively a much easier target for gene therapy.
- One more basic reason for gene therapy for Hemophilia being so encouraging is that even minimal success from the gene therapy procedure can mean a significant improvement in quality of life for patients. In some peculiar tests, this therapy has led to a marginal improvement in clotting factors in the blood, and also the time it took for the subject’s blood to clot reduced remarkably. People who experience such kind of improvements after gene therapy may be able to go for longer time durations without supplemental clotting factors infusions.
However, gene therapy for Hemophilia is not completely positive, given the lack of people participating in clinical studies. Some of the essential setbacks include
- The results of gene therapy for Hemophilia treatment are proven to be inconsistent. Many studies that are conducted on animals have procured good results often, but that still hasn’t managed to translate similar results in human beings.
- Another con is the factor that there is a severe risk to the patient’s body while undergoing gene therapy for Hemophilia treatment. It is observed that the patient will develop antibodies to the viral vectors used to deliver the genetic material to the cells.
- The method of treatment is another setback when it comes to gene therapy for Hemophilia. For the genetic material to go inside the cellular structure, it is essential to attach it to viruses, which are then injected inside the body where they can invade those cells. In some cases, an immune response or reaction can be triggered due to this. The patient’s body in return can produce antibodies that can destroy the particular viruses, stopping the treatment from happening in the first place.
- Taking the decision of whether to undergo gene therapy or not can be extremely difficult. Apart from the insurance questions, the biggest barrier is that gene therapy results aren’t guaranteed and if it doesn’t work in the favour, patients will never get another chance for using the same therapy or a similar vector for re-treatment.
In conclusion, gene therapy has an excellent safety profile, but the fact that only around 100 people with Hemophilia have undergone treatment whose results were taken under consideration. Many adverse reactions are expected to occur when several thousands of people may actually undergo this gene therapy treatment. Unfortunately, scientists are still not able to predict whether the transmitted gene copy or the expressing cells will be stable and the results will persist, or if they may be inactivated which means a patient is back to square one.
What Does the Future Hold for Gene Therapy for Hemophilia
FDA has promised to accelerate the reviews of gene therapies for Hemophilia A and B. DelveInsight analysts anticipate that these therapies could have a quoted price of $2.5 to $3 million, making them the most expensive drugs ever to reach the market. With vigorous research, inclusion parameters can be broadened and gene therapy can be available to people with less severe diseases. Scientists are looking at other viral vectors, including – lentivirus, which can open up gates of gene therapy to people who have antibodies associated with Adeno-Associated Virus (AAV).
For more than a decade now, gene therapy is considered to be one of the most sought-after treatments for Hemophilia. A one-shot targeted process that can fix the inherited condition at its source and operates on replacement of a defective gene with a functional one to produce sufficient clotting factor. Now that this revolutionary treatment is assured to become very much of a reality, several significant pharmaceutical companies and drug giants are expected to receive US Food and Drug Administration approval to market gene therapy for Hemophilia in the subsequent years.
Frequently Asked Questions
Yes, they may vary as a response to gene therapy. Solely based on the clinical trials, factors may be moderate, mild or normal Hemophilia range and may fluctuate over time. Whether there is a requirement of factors if you have an accident, injury or require surgery will depend on the factor level.
No, it is not possible to reverse or undo the one-time intravenous infusion used for gene therapy.
No, gene therapy for Hemophilia corrects the genetic defect only in the person who receives it. It does not correct the genes that are passed onto the next generation.
Some of the major pharmaceuticals working proactively in the field of gene therapy for Hemophilia include names like Spark Therapeutics, Sigilon Therapeutics, ASC Therapeutics, Pfizer, Sanofi Genzyme, Novo Nordisk, UniQure Biopharma B.V./ CSL Behring, Sangamo Therapeutics, Pfizer, Genzyme, ApcinteX Ltd, and Freeline Therapeutics.
Downloads
Article in PDF
Recent Articles
- DelveInsight’s Hematological disorders based Gene Therapy Reports
- Hemophilia B Market: How Pipeline Therapies are Transforming the Treatment Hemisphere?
- Global Hemophilia Market
- Hemophilia: A Lifelong Genetic Disorder
- Daiichi Sankyo’s Ezharmia; Pfizer & Sangamo Hemophilia A Gene Therapy Trial; Approval for Fe...