Giant-Cell Arteritis Treatment Beyond Glucocorticoids: Exploring Horizons
Mar 11, 2024
Giant-cell arteritis (GCA), alternatively referred to as temporal arteritis, is a form of vasculitis primarily impacting arteries, particularly those in proximity to the temples. If left untreated, it may result in notable complications such as loss of vision. Diagnosis of the disease is often complicated by its protean presentation and lack of consistently reliable testing. Novel techniques for risk assessment with Halo scoring and stratification through axillary vessel ultrasound are becoming commonplace.
Giant cell arteritis stands as the predominant form of vasculitis among individuals aged 50 and above. It manifests through granulomatous inflammation affecting medium to large arteries, notably the temporal artery, and presents with acute symptoms such as headache, claudication, and visual impairments.
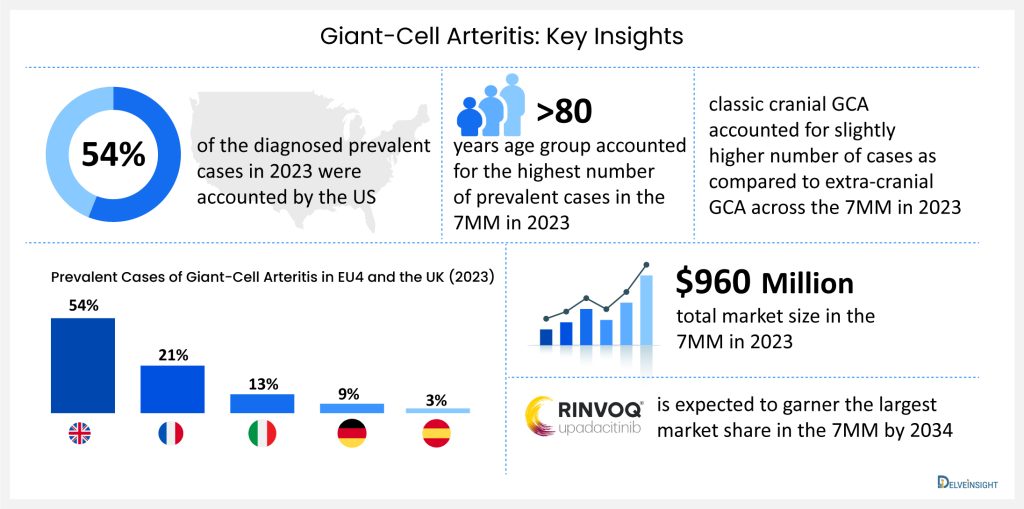
According to DelveInsight’s analysis, in 7MM, the United States accounted for the highest number of diagnosed prevalent cases of giant-cell arteritis, which is 54% of the diagnosed prevalent cases of giant-cell arteritis in 2023. These numbers are projected to rise by 2034 due to aging populations worldwide and improved diagnostic capabilities.
Downloads
Article in PDF
Recent Articles
- Zevra’s MIPLYFFA Niemann-Pick Disease Approval; FASENRA Approved for Eosinophilic Granulomatosis;...
- Merck’s Gefapixant; Pfizer’s Somatrogon; Gilead’s Viklury; AbbVie’s Skyrizi; Gilead’s...
- HUMIRA Biosimilars in the US: The Talk of the Psoriatic Arthritis Treatment Market
- Ipsen and Skyhawk Therapeutics Partnership; SynOx Therapeutics’ Phase III Trial; Roche’s Alecensa...
In 2023, as far as GCA cases by subtype are concerned, classic cranial GCA accounted for slightly higher number of cases as compared to extra-cranial GCA across the 7MM. It was also observed that across the 7MM, patients in the age segment >80 years accounted for the highest number of prevalent cases of GCA in 2023. As far as cases by clinical manifestations of GCA are concerned, scalp tenderness accounted for the highest number of cases whereas, vision loss accounted for the least number of cases across the 7MM in 2023.
The primary aim for giant-cell arteritis treatment is to prevent permanent vision loss and to control the inflammation of the blood vessels that can lead to tissue damage. When a doctor suspects a patient may have giant-cell arteritis, treatment should commence right away, even if the diagnosis has not been confirmed by tests. The primary treatment for giant-cell arteritis involves using corticosteroids. Even in cases where the diagnosis is only suspected and test results are pending, doctors may prescribe oral corticosteroids promptly. Prednisone stands out as the most commonly prescribed corticosteroid, known for its effectiveness in preventing vision loss. The response to prednisone treatment is usually quite remarkable, with improvements in blood markers of inflammation often seen within 2-4 weeks.
To address giant-cell arteritis, physicians may recommend a high corticosteroid dosage ranging from 40 mg to 60 mg per day, typically lasting for about 3 to 4 weeks. If there is improvement in the patient’s condition, the dosage is then gradually decreased. However, there has been ongoing debate regarding the safety profile of corticosteroids. Prolonged use of corticosteroids can lead to significant, sometimes inevitable side effects such as osteoporosis, muscle weakness, cataracts, glaucoma, diabetes, and high blood pressure. Due to these adverse effects, it is crucial to explore safer alternative treatments. The potential side effects of corticosteroids are also driving the use of tocilizumab and the investigation of other medications in clinical trials to mitigate their toxicity.
Currently, there is just one therapy that is approved in the 7MM for giant-cell arteritis treatment, which includes Chugai Pharmaceuticals/Roche’s ACTEMRA/ROACTEMRA (tocilizumab). ACTEMRA was granted FDA approval in May 2017, marking a significant milestone as the first drug approved for adults with GCA. This decision was informed by the favorable results of the Phase III GiACTA study, which assessed the effectiveness of ACTEMRA in GCA patients. Before its approval, the FDA had awarded ACTEMRA Priority Review status and Breakthrough Therapy Designation specifically for its use in GCA.
ACTEMRA represents an innovative anti-IL-6 receptor (aIL-6R) therapy, pioneering a new class of treatments. IL-6 is recognized as a pivotal player in triggering the inflammatory process underlying the manifestations of rheumatoid arthritis and similar autoimmune disorders. By binding to the IL-6 receptor, ACTEMRA effectively hinders the action of the inflammatory protein IL-6. This intervention leads to a reduction in joint pain, swelling associated with arthritis, and alleviates other inflammation-induced symptoms. Additionally, its approval extends to treating pediatric juvenile idiopathic arthritis (pJIA), systemic juvenile idiopathic arthritis (sJIA), as well as cytokine release syndrome (CRS) induced by CAR-T cell therapy.
In November 2018, Roche declared that the ACTPen 162 mg/0.9 mL, a prefilled autoinjector designed for single use, received approval from the FDA. This approval pertains to its use as an alternative formulation of ACTEMRA for treating giant-cell arteritis in adult patients.
Productive pharmacologic options for managing the most prevalent and most disabling phases of giant-cell arteritis are minimal. Treatments that work in this disorder are scarce; therefore, new giant-cell arteritis treatments are desperately needed. Some companies like Novartis (COSENTYX; secukinumab), AbbVie (RINVOQ; upadacitinib), and J&J/MorphoSys AG (TREMFYA; guselkumab) have initiated clinical trials that investigate new giant-cell arteritis treatment options.
RINVOQ (upadacitinib) is an oral medication that selectively and reversibly inhibits Janus kinase (JAK), a crucial component in the immune signaling pathway, particularly the JAK-STAT pathway utilized by proinflammatory cytokines to communicate with the cell nucleus. When these signaling pathways malfunction, they provoke an exaggerated inflammatory response, manifesting as persistent pain, swelling, and gradual joint deterioration. RINVOQ has received approval for use in the United States, Japan, and the European Union. It is designated for treating moderate to severe active rheumatoid arthritis in adults who have not adequately responded to or cannot tolerate one or more disease-modifying anti-rheumatic drugs (DMARDs). Abbvie is the pharmaceutical company overseeing its development.
JAKs are enzymes located inside cells that convey signals from interactions of cytokines or growth factor receptors on the cell membrane, affecting processes related to hematopoiesis and the function of immune cells. In this signaling process, JAKs add phosphate groups to and activate Signal Transducers and Activators of Transcription (STATs), which then regulate activities within the cell, such as gene expression. RINVOQ intervenes in this pathway at the JAKs stage, preventing the phosphorylation and subsequent activation of STATs. Presently, RINVOQ is undergoing Phase III clinical trials for giant-cell arteritis treatment.
COSENTYX (secukinumab) is an injectable monoclonal antibody derived from humans, designed to specifically block interleukin-17A (IL-17A), a cytokine linked to various immune-related conditions. Developed by Novartis Pharmaceuticals, it has received approval in the United States and European Union for the treatment of patients with moderate-to-severe plaque psoriasis. Additionally, it is approved for adults with active ankylosing spondylitis, adults with active non-radiographic axial spondyloarthritis (nr-axSpA), and adults with active psoriatic arthritis.
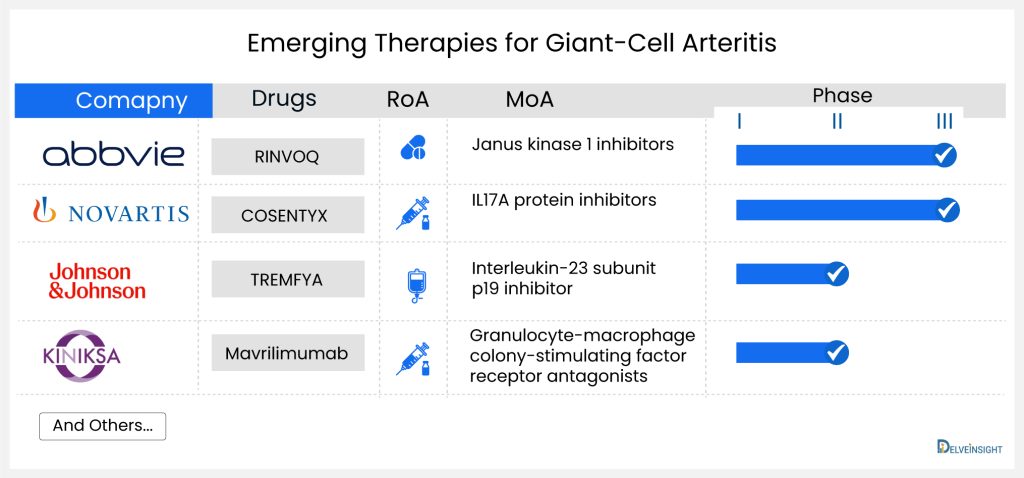
Secukinumab, a monoclonal antibody of the human IgG1 type, specifically attaches to the interleukin-17A (IL-17A) cytokine, preventing its interaction with the IL-17 receptor. IL-17A is a naturally occurring cytokine crucial in normal immune and inflammatory responses. By targeting this cytokine, Secukinumab hinders the release of proinflammatory cytokines and chemokines. The company has finished a Phase II trial and is currently advancing through Phase III clinical development for giant-cell arteritis treatment. The trial (NCT04930094) has been initiated and is actively enrolling participants.
TREMFYA (guselkumab) is a human monoclonal antibody against the p19 subunit of interleukin (IL)-23 developed by Janssen. Currently, TREMFYA is undergoing a Phase II clinical trial for giant-cell arteritis treatment.
The emergence of potential therapies is expected to provide a boost to market growth, DelveInsight estimates that the market size for giant-cell arteritis is expected to grow from USD 960 million in 2023 with a significant by 2034. As per the estimates, RINVOQ (upadacitinib) is expected to garner the largest giant-cell arteritis market share in the 7MM by 2034. The growth of the giant-cell arteritis market is expected to be mainly driven by increased diagnosed incidence, patient awareness, and a robust clinical pipeline during the forecast period (2024–2034).
The improved and clear treatment and diagnostic guidelines have led patients and practitioners to clearly diagnose and treat patients accurately, while glucocorticoids remain the primary treatment. Ongoing research into alternative and adjunctive therapies, such as biologic agents like tocilizumab, provides more tailored approaches for patients, considering factors like side effects and individual response, and offers a significant opportunity for improving treatment outcomes in GCA. Biologics, such as interleukin-6 (IL-6) inhibitors like tocilizumab, have shown promise in clinical trials, and further research and development in this area can lead to more effective and targeted treatments. The introduction of biosimilars for existing therapies can increase competition, potentially reducing treatment costs and improving accessibility for patients.
In a nutshell, a few potential therapies are being investigated for the management of giant-cell arteritis. Even though it is too soon to comment on the above-mentioned promising candidate to enter the giant-cell arteritis treatment market during the forecast period, it is safe to assume that the future of this market is bright. Eventually, the drug shall create a significant difference in the landscape of giant-cell arteritis treatment in the coming years. The giant-cell arteritis treatment space is expected to experience a positive impact in the coming years owing to the improvement in the rise of healthcare spending across the world.

Downloads
Article in PDF
Recent Articles
- Merck’s Gefapixant; Pfizer’s Somatrogon; Gilead’s Viklury; AbbVie’s Skyrizi; Gilead’s...
- Ipsen and Skyhawk Therapeutics Partnership; SynOx Therapeutics’ Phase III Trial; Roche’s Alecensa...
- Zevra’s MIPLYFFA Niemann-Pick Disease Approval; FASENRA Approved for Eosinophilic Granulomatosis;...
- HUMIRA Biosimilars in the US: The Talk of the Psoriatic Arthritis Treatment Market