Glioma vs. Glioblastoma Therapeutics Space: Unveiling the Battlefront
Oct 11, 2024
Table of Contents
Glioma and glioblastoma are both types of brain tumors, but they differ significantly in their characteristics and prognosis. Glioma is a broad term that encompasses tumors originating from glial cells, which are supportive cells in the brain. These tumors can vary in grade and aggressiveness, with some growing slowly (low-grade gliomas) and others more rapidly (high-grade gliomas). Glioblastoma, on the other hand, is the most aggressive and malignant form of glioma. It is classified as a grade IV tumor and is known for its rapid growth and invasive nature. Glioblastoma is notorious for its resistance to treatment and poor prognosis, with most patients surviving only around 15 months after diagnosis despite aggressive therapy including surgery, radiation, and chemotherapy.
While glioma and glioblastoma share the same origin in the brain’s glial cells, their behavior and clinical outcomes are markedly different. Glioma encompasses a spectrum of tumors that can vary in terms of grade, location, and response to treatment. Some gliomas, particularly low-grade varieties, may have a relatively favorable prognosis and respond well to treatment, allowing for extended survival and improved quality of life. However, glioblastoma stands out for its highly malignant nature, characterized by rapid growth, infiltrative behavior into surrounding brain tissue, and resistance to standard treatments. Despite advancements in medical technology and therapies, glioblastoma remains one of the most challenging brain tumors to treat, highlighting the urgent need for continued research and innovation in this field.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Notizia
- First Gene Therapy for Severe Hemophilia A; FDA Approves CellTrans’s Type 1 Diabetes Cellular The...
- Phase III RUBY Trial of Jemperli Plus Chemotherapy Updates; FDA Approves Roche’s Vabysmo for RVO;...
- Glioblastoma Multiforme: Advancements in the Treatment Paradigm of the Malignant Condition
- Biogen terminates ALS Pact with Karyopharm; AbbVie’s Immunological Drug Skyrizi; NICE Backs Astel...
Understanding the Epidemiology: Glioma and Glioblastoma Incidence Patterns
Glioblastoma incidence is relatively lower compared to other gliomas but carries a disproportionately grave impact due to its aggressive behavior and limited treatment options. Consequently, despite its lower incidence, glioblastoma accounts for a significant portion of brain tumor-related morbidity and mortality.
According to DelveInsight analysis, the total incident cases of glioma in the 7MM comprised ~42K cases, with the United States accounting for 40% of the total, or approximately 19,100 cases. Grade IV gliomas led the way in the US, with around 13,200 cases, while Grade I had the fewest. The highest incidence was observed in the 60–74 years age group (36%), followed by the 45–59 years group (25%). Glioblastoma was the most prevalent type, contributing 67% of cases, followed by diffuse and anaplastic astrocytomas (each at 8%).
In 2023, the US recorded the highest number of incident glioblastoma cases among the 7MM, with approximately 13,600 cases. Within the EU4 and the UK, Germany had the most incident cases, followed by France. Specifically in the US, there were about 12,200 cases of Primary GBM/IDH-wild Type and 1,360 cases of Secondary GBM/IDH Mutant. According to DelveInsight, the 65–74 years age group accounted for the highest number of cases, followed closely by those aged 55–64 years.
Current Glioma and Glioblastoma Therapeutic Approaches
Despite advancements in therapy, the prognosis for patients remains poor, necessitating ongoing research into novel therapeutic approaches. Current therapeutic strategies for gliomas often involve a multi-modal approach, combining surgery, radiation therapy, and chemotherapy. Surgery aims to remove as much of the tumor as possible, alleviating symptoms and reducing tumor burden. However, due to the infiltrative nature of gliomas, complete resection is often unattainable, leading to tumor recurrence.
Radiation therapy, delivered post-surgery, targets remaining tumor cells, aiming to destroy them or impair their ability to proliferate. While effective in reducing tumor size and delaying progression, radiation therapy can also damage healthy brain tissue, leading to debilitating side effects. Chemotherapy, particularly with the alkylating agent temozolomide, is the accepted therapy for high-grade gliomas (HGG) in adults, but there’s no universally recognized standard chemotherapy for pediatric HGG. Specifically for diffuse intrinsic pontine glioma (DIPG), chemotherapy hasn’t been firmly established, with radiation being the primary treatment. However, it’s largely palliative, as less than 10% of children survive beyond 2 years, despite many experiencing temporary relief from neurological symptoms after radiotherapy. Therefore, there is a continuous search for new chemotherapeutic agents and combination therapies to overcome resistance and improve outcomes.

Immunotherapy represents a cutting-edge and hopeful avenue for treatment, aiming to activate the body’s own immune system to combat and stop the progression of tumors. Specifically, immunotherapy, often referred to as “vaccine” therapy, works by stimulating the immune system to target and attack individual tumors. This approach commonly utilizes the patient’s own tumor cells as the immune stimulant. Additionally, Gliadel Wafer, an alkylating drug, is prescribed for the treatment of newly diagnosed high-grade glioma alongside surgery and radiation, and for recurrent glioblastoma multiforme (rGBM) alongside surgery.
In March 2023, Novartis unveiled an extension of the approved usage of TAFINLAR (dabrafenib) + MEKINIST (trametinib). This expansion now includes the treatment of low-grade glioma in pediatric patients aged 1 year and above who possess a BRAF V600E mutation. This supplement augments its existing authorization to address low-grade glioma in both adult and pediatric patients aged 6 years and older.
On August 6, 2024, the FDA approved vorasidenib for patients with Grade 2 gliomas harboring IDH1 or IDH2 mutations. This approval was supported by results from the INDIGO clinical trial, a global Phase 3, double-blinded, randomized study. The trial showed that vorasidenib more than doubled progression-free survival and significantly delayed the need for radiation and chemotherapy in patients with Grade 2 IDH-mutant glioma following tumor removal surgery. INDIGO also marked the first-ever Phase 3 clinical trial for a molecularly targeted therapy in IDH-mutant glioma.
The management of gliomas, including glioblastoma, remains a complex and evolving field. While current therapeutic approaches provide some benefit, the quest for more effective and personalized treatments continues unabated. Collaborative efforts between researchers, clinicians, and pharmaceutical companies are essential to translate promising preclinical findings into clinically meaningful outcomes for patients with gliomas.
Glioma and Glioblastoma Market Players and Pipeline Analysis: Who’s Leading the Charge?
The glioma and glioblastoma market landscape is characterized by dynamic research efforts and the pursuit of innovative therapeutic strategies aimed at improving patient outcomes. With a diverse array of market players and a robust pipeline of investigational therapies, there is cautious optimism that the future holds promising advancements in the management of these challenging brain tumors.

Emerging Therapies for Glioma Treatment
The dynamics of the glioma treatment market are anticipated to change in the coming years owing to the positive outcomes of the emerging pipeline candidates. Some key players targeting H3 K27 mutation are Chimerix (ONC201), Bexion (BXQ-350), and others. Other players developing therapies for HGG treatment include MimiVax (SurVaxM), Orbus Therapeutics (eflornithine), Aivita Biomedical (AV-GBM-1), and others. A few of the emerging therapies for glioma treatment being developed by these key players are explained below:
ONC201, also known as dordaviprone, is a novel small molecule imipridone that specifically targets the G-protein coupled dopamine receptor D2 (DRD2) and the mitochondrial protease ClpP. Its registration program concentrates on patients with brain tumors carrying the H3 K27M mutation due to its notable and enduring positive responses in cases of recurrence. The FDA has expedited the development of ONC201 by granting it fast-track designation for treating adult recurrent H3 K27M-mutant high-grade glioma, rare pediatric disease designation for H3 K27M-mutant glioma, and orphan drug designations for glioblastoma and malignant glioma. In November 2021, an analysis combining safety and effectiveness data of ONC201 as a standalone treatment for recurrent H3 K27M-mutant diffuse midline glioma revealed a disease control rate of 40%, with progression-free survival rates of 35% at 6 months and 30% at 12 months. The Phase III ACTION trial, presently recruiting participants across 77 sites in 11 countries, is expected to provide interim results in early 2025 as planned. In August 2023, an international team of researchers, led by the University of Michigan Health Rogel Cancer Center and the Chad Carr Pediatric Brain Tumor Center, made a groundbreaking discovery regarding the drug candidate ONC201. This compound has shown promise in improving survival rates for patients with diffuse midline gliomas (DMG), including the aggressive diffuse intrinsic pontine glioma (DIPG), for which there are currently no effective treatments. The findings, published in the journal Cancer Discovery, revealed that ONC201 nearly doubled survival compared to previous patients, marking a significant advancement in the fight against this challenging brain tumor.
BXQ-350 (Bexion), a new biological substance, is undergoing examination in both adult and pediatric cancer populations. It works by regulating sphingolipid metabolism, elevating ceramide levels, and reducing sphingosine-1-phosphate (S1P). Findings from a prior study in pediatric patients demonstrated favorable tolerance of BXQ-350, with its safety profile encouraging further exploration at the highest tested dosage. Presently, it is undergoing Phase I trials.

SurVaxM, developed by MimiVax, represents a pioneering patented peptide mimic immunotherapeutic vaccine designed to combat survivin, a protein vital for cell survival found in 95% of glioblastoma and various other cancers. This vaccine operates through two distinct mechanisms: it activates the patient’s T-cell immunity while also utilizing antibody-directed inhibition to disrupt the survivin pathway, thereby restraining tumor growth and potentially impeding tumor recurrence. MimiVax is presently enrolling participants for a Phase IIb trial. Notably, in October 2023, the US FDA granted fast-track designation to MimiVax’s SurVaxM vaccine, currently under investigation for the treatment of newly diagnosed glioblastoma.
In May 2024, MimiVax Inc. received a supplemental Orphan Drug Designation from the FDA for SurVaxM vaccine, now including malignant glioma. The following month, the FDA expanded the designation to cover SurVaxM for all malignant gliomas in both children and adults, broadening its use beyond just adult glioblastoma.
Emerging Therapies for Glioblastoma Treatment
Some of the drugs in the glioblastoma pipeline include BMX-001 (BioMimetix), EO2401 (Enterome), LAM561 (Laminar Pharmaceuticals), MDNA55 (Medicenna), Temferon (Genenta Science), and others. LAM561, also known as 2-hydroxylic acid, is a synthetic variant of oleic acid designed for oral administration with the potential to penetrate the Blood-Brain Barrier, reaching brain cells. This compound modifies the composition of cancer cell membranes, thereby reducing the activity of signaling proteins associated with tumor growth. Initial trials of LAM561 demonstrate promising outcomes in treating aggressive brain tumors like glioblastomas. Currently undergoing Phase II/III evaluation, LAM561 is anticipated to gain approval for adult glioma treatment in 2024, followed by potential approval for pediatric glioma treatment by 2025/2026, according to the company’s development timeline.
In June 2024, Laminar Pharmaceuticals completed patient enrollment for the CLINGLIO trial, which evaluates idroxioleic acid (LAM561) in treating newly diagnosed glioblastoma. The Phase IIb/III trial, involving 140 adults across Spain, Italy, France, and the UK, combines LAM561 with standard care (tumor resection and chemoradiotherapy). This novel, synthetic fatty acid, administered orally, represents a promising new therapeutic approach for this aggressive brain cancer.
BMX-001, a metalloporphyrin, belongs to a new category of small molecules with redox-active properties. Engineered to replicate the active site of superoxide dismutase, its primary function involves regulating cellular signaling pathways. Currently undergoing Phase II trials for individuals with recently diagnosed high-grade glioma, BMX-001 garnered Orphan Drug Designation (ODD) in January 2020 and Fast Track Designation (FTD) in May 2020. In 2022, the FDA granted Breakthrough Therapy Designation (BTD) for BMX-001, particularly for its potential combined use with standard radiotherapy and temozolomide in newly diagnosed high-grade glioma cases.
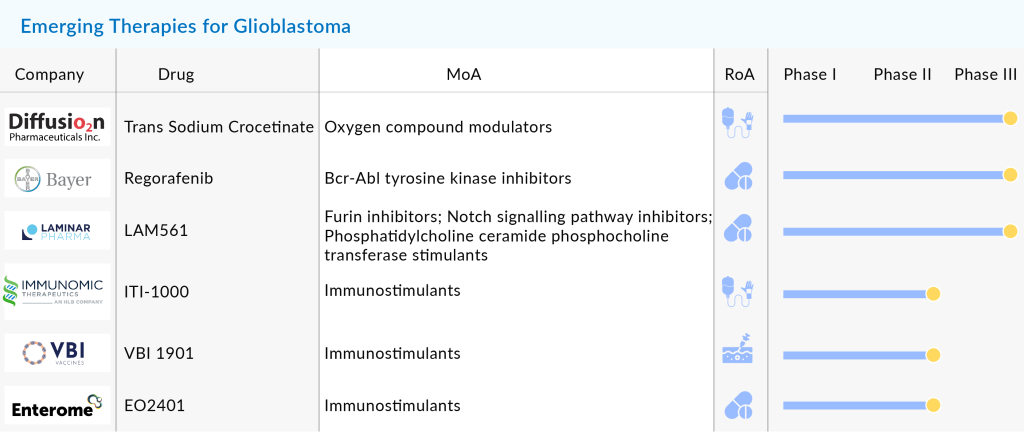
EO2401 consists of three bacterial peptides known as OncoMimics, which closely resemble three crucial human tumor antigens found in glioblastoma and adrenal tumors. The compound is currently undergoing assessment alongside a checkpoint inhibitor (nivolumab) for glioblastoma treatment in a Phase Ib/IIa trial named ROSALIE. Recently, in November 2023, the company disclosed enhanced efficacy results from the ROSALIE trial, revealing an 18-month survival rate of 43%. This development has prompted the company to pursue establishing a pathway for EO2401’s registration.
Treatment Market Trends and Future Prospects: A Glimpse into Tomorrow
In recent years, the landscape of glioma and glioblastoma treatment has been witnessing notable shifts driven by advancements in medical technology, increased understanding of tumor biology, and the emergence of novel therapeutic approaches. One of the prominent trends in the glioma and glioblastoma market is the growing emphasis on precision medicine and personalized treatment strategies. With the advent of genomic profiling and molecular diagnostics, clinicians are increasingly able to tailor therapies based on the specific genetic alterations and molecular characteristics of individual tumors. This trend not only enhances treatment efficacy but also minimizes the likelihood of adverse reactions, leading to improved patient outcomes.

As per DelveInsight’s analysis, the total market size of glioblastoma across the 7MM reached approximately USD 1.9 billion in 2023. Meanwhile, the total market size of glioma in the 7MM was around USD 1 billion in 2023. These figures highlight the growing market for both glioblastoma and glioma treatments.
The glioma and glioblastoma treatment market is witnessing a surge in the development of targeted therapies and combination treatment regimens. Small molecule inhibitors targeting specific signaling pathways implicated in tumor growth and survival, such as the EGFR and PI3K pathways, are being investigated either as monotherapies or in combination with standard treatments like chemotherapy and radiation therapy. Additionally, efforts to explore synergistic effects between different therapeutic modalities, such as immunotherapy and radiotherapy, hold promise for improving treatment outcomes and overcoming resistance mechanisms.
Looking ahead, the future prospects for the glioma and glioblastoma market are buoyed by continued research and innovation across multiple fronts. Advances in technologies such as artificial intelligence and machine learning are expected to enhance the identification of novel therapeutic targets and the prediction of treatment responses. Moreover, ongoing clinical trials exploring innovative treatment modalities, including gene therapies, oncolytic viruses, and nanoparticle-based drug delivery systems, offer potential avenues for addressing unmet medical needs in this challenging disease landscape.

Downloads
Article in PDF
Recent Articles
- First Gene Therapy for Severe Hemophilia A; FDA Approves CellTrans’s Type 1 Diabetes Cellular The...
- Daiichi Sankyo’s Intravenous Iron Replacement Therapy; ANeuroTech’s Adjunctive Anti-depression Dr...
- CAR-T in killing tumors; Amunix raises $73M; Autism findings in protein-targeted treatment
- Merck’s Gefapixant; Pfizer’s Somatrogon; Gilead’s Viklury; AbbVie’s Skyrizi; Gilead’s...
- Glioblastoma Multiforme: Advancements in the Treatment Paradigm of the Malignant Condition



