Unveiling the Future: Global Neuroendocrine Tumor Market Trends & Innovations
Oct 30, 2024
Neuroendocrine tumors (NET) begin in the neuroendocrine system of the body, developing in cells that interact with neurons and hormone-producing cells. These cells can be found throughout the body, but most often in the abdomen, particularly in the gastrointestinal tract. The tumor can also develop in the lungs, pancreas, and adrenal glands. In about 15% of cases, the primary site of the neuroendocrine tumor is not identified.
Neuroendocrine tumor symptoms can vary widely depending on the tumor’s location and the hormones it produces. While some neuroendocrine tumors are slow-growing and may not cause noticeable symptoms, others can lead to significant health issues, highlighting the importance of early diagnosis and effective neuroendocrine tumor treatments.
Neuroendocrine tumors are classified based on different criteria like Anatomic site of origin, including Gastrointestinal NETs, Pancreatic NETs, Lung NETs, and others (medullary carcinoma, Merkel cell carcinoma, thymic neuroendocrine cancer, and paraganglioma), if the Symptoms are Functional and non-functional if the tumor is Malignant (cancerous) and non-malignant (non-cancerous), Grades of the tumors and finally based on Stages of the tumor either Localized, Regional or Distant.
Downloads
Article in PDF
Recent Articles
- Understanding Neuroendocrine Tumors (NETs): An In-Depth Look
- Gastrointestinal Cancers: Exploring the Range of Digestive Tract Malignancies
- From Beta to Alpha Emitters: Radioligand Therapies Reshaping the Neuroendocrine Tumors Treatment ...
- Neuroendocrine Tumors (NETs): Unraveling the Mystery of a Complex Cancer
The most common symptoms experienced by a NET patient include fatigue, loss of appetite, uncontrolled and unexplainable weight loss, persistent pain in the affected area, nausea, vomiting, unusual bleeding, diarrhea, facial flushing, and skin rashes. If the NET is functional, it expresses itself by releasing a hormone called serotonin, leading to symptoms such as diarrhea and facial flushing, which can make diagnosis easier.
In contrast, non-functional NETs do not release enough serotonin, making them harder to diagnose. Approximately 70% of neuroendocrine tumor cases are functional, while the remaining 30% are non-functional, often resulting in diagnoses at more advanced stages. Early recognition of neuroendocrine tumor symptoms is crucial for improving treatment outcomes.
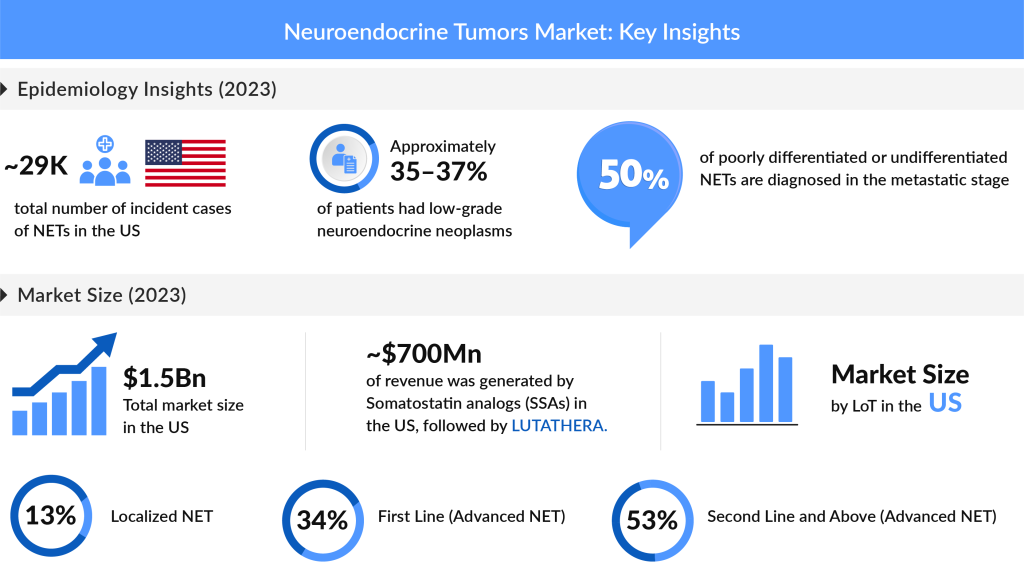
Neuroendocrine Tumors are rare. However, during the past decade, the incidence of NETs has been increasing. In 2023, the total number of incident NET cases in the US reached nearly 29K, with projections indicating further increases. Non-functional, asymptomatic, or unknown NETs accounted for the majority of these cases, with approximately 16,000 reported, compared to about 15K for functional NETs. Among the EU4 countries and the UK, the UK had the highest incidence, representing nearly 30% of the total NET cases in the region for the same year.
Neuroendocrine Tumor Therapy Market
The treatment landscape for neuroendocrine tumors has expanded remarkably in recent years, providing more tailored solutions for patients facing this complex neuroendocrine tumor cancer. Surgery remains a primary option, especially for localized NETs; however, advances in neuroendocrine tumor treatments now include sophisticated nonsurgical options for those with advanced disease. These newer treatments—such as hepatic artery embolization (HAE), selective internal radiotherapy (SIRT), radiofrequency ablation (RFA), and palliative hepatic cytoreductive surgery—help reduce tumor burden and manage symptoms, particularly for patients with liver metastases. For neuroendocrine tumors expressing somatostatin receptors (SSTR), long-acting somatostatin analogs (SSAs) are highly effective for symptom control and hormone suppression, improving quality of life and progression-free survival.

The range of FDA-approved therapies for neuroendocrine tumor treatments continues to grow, enhancing the therapeutic options for patients with various NET types, including pancreatic neuroendocrine tumors. Key therapies include LUTATHERA (lutetium Lu 177 dotatate), SOMATULINE DEPOT (lanreotide), AFINITOR (everolimus), and AZEDRA (iobenguane I-131), each addressing different needs within the NET community. Notably, LUTATHERA has brought a new approach with Peptide Receptor Radionuclide Therapy (PRRT), initially approved for adult NET patients and more recently for pediatric patients (ages 12 and up) with SSTR-positive gastro-entero-pancreatic neuroendocrine tumors (GEP-NETs). This PRRT therapy, based on its robust data, showcases improved efficacy in treating well-differentiated NETs.
With these advancements in neuroendocrine tumor treatments, clinicians now have more options to manage neuroendocrine tumor symptoms, allowing for greater adaptability in addressing unique patient needs, from disease stabilization to symptom relief.
We can Expect a Better NET Treatment Future
The landscape for neuroendocrine tumor treatments is undergoing an exciting evolution, with somatostatin analogs (SSAs) maintaining a key role as the standard of care for many patients. Besides their well-known antisecretory effects, these therapies exhibit antiproliferative properties that help slow disease progression. Recent studies have shown that SSAs like Octreotide and Lanreotide significantly improve both time to tumor progression and overall survival compared to placebo.
However, as therapeutic demands shift, targeted therapies are playing an increasingly pivotal role. The mTOR inhibitor everolimus is widely used, especially for tumors with rapid progression or when somatostatin receptor imaging is negative. Likewise, Peptide Receptor Radionuclide Therapy (PRRT), such as LUTATHERA, has been established as a novel option for patients who are refractory to other treatments. Nonetheless, for patients with advanced neuroendocrine tumors, particularly metastatic disease, prognosis remains challenging, highlighting a pressing need for further advancements in the therapeutic pipeline.
Guidelines from both the NCCN and ENETS suggest SSAs as first-line therapies in cases of low-grade tumors or slow progression rates where SSTR-related imaging is positive. For higher-grade tumors or rapidly progressing cases, everolimus is often recommended as either first-line or second-line therapy. For NETs originating in the lungs, additional options such as chemotherapy, PRRT, locoregional treatments, and experimental therapies in clinical trials may be considered after SSA and everolimus therapy.
The total US market size for NETs was estimated at around USD 1.53 billion in 2023, with somatostatin analogs and LUTATHERA capturing a significant share. In the EU4 and the UK, the UK market alone accounted for nearly 31% of the NET market. The demand for innovative approaches is high, with radiopharmaceutical developers prioritizing supply capabilities to address unmet needs among neuroendocrine tumor cancer patients.

The pipeline for neuroendocrine tumor therapies is equally promising, with notable updates from several companies. In April 2024, Perspective Therapeutics‘ investigational agent [212Pb]VMT-a-NET was selected for the FDA’s readiness pilot program, aiming to fast-track manufacturing for NET treatments. Additionally, Crinetics Pharmaceuticals announced plans to initiate a Phase III program of paltusotine for carcinoid syndrome by late 2024. Furthermore, Camurus expects topline results for CAM2029 in early 2025, and Bristol Myers Squibb (BMS) is advancing RYZ101 as a second-line option for GEP-NETs with an anticipated data release in 2026. The recent BMS acquisition of RayzeBio underscores the importance of Radiopharmaceutical Therapies (RPTs) in the evolving NET landscape, specifically through RayzeBio’s RYZ101, which targets SSTR2 in GEP-NETs and ES-SCLC. Looking ahead, these advancements represent a transformative period in NET therapies, as the introduction of new therapies could enhance both patient outcomes and quality of life.
In the fast-evolving realm of neuroendocrine tumor treatments, the therapeutic horizon is expanding with remarkable potential to change patient outcomes. As new drugs and targeted therapies like PRRT and everolimus pave the way for more effective treatments, hope is growing for improved quality of life, especially for those with advanced neuroendocrine tumors. With industry leaders making strides in drug development and regulatory approvals, such as Bristol Myers Squibb’s RYZ101 and Crinetics Pharmaceuticals’ paltusotine, patients now have a promising future with more tailored and potent options on the horizon.
This shift underscores a meaningful transformation in NET care—where emerging innovations may soon become the cornerstone of a more hopeful prognosis for patients worldwide.

Downloads
Article in PDF
Recent Articles
- Understanding Neuroendocrine Tumors (NETs): An In-Depth Look
- From Beta to Alpha Emitters: Radioligand Therapies Reshaping the Neuroendocrine Tumors Treatment ...
- Gastrointestinal Cancers: Exploring the Range of Digestive Tract Malignancies
- Neuroendocrine Tumors (NETs): Unraveling the Mystery of a Complex Cancer