HPK1 Inhibitors: A Promising Frontier in Immuno-Oncology Therapeutics
Dec 29, 2023
Table of Contents
Hematopoietic progenitor kinase (HPK1) belongs to the family of Ste20 serine/threonine kinases known as mitogen-activated protein kinase kinase kinase kinase (MAP4K). It is primarily expressed in hematopoietic cells and functions as a negative regulator of T-cell receptor (TCR) and B-cell signaling, serving as an immune checkpoint. Mice with a kinase-dead mutation in HPK1 exhibit enhanced CD8+ T cell activity, increased secretion of cytokines, and potent anti-tumor immune responses, even within an immunosuppressive tumor microenvironment. Consequently, HPK1 emerges as a promising target in the field of immuno-oncology.
While studies involving the knockout of the HPK1 gene suggest its contribution to anti-tumor immune responses, the specific role of its kinase activity and its potential for therapeutic applications remain unclear. Cell biological investigations indicate that HPK1 is responsive to activation signals from various cell surface receptors found on different hematopoietic cell subtypes. These receptors include TCR, BCR, Fas, EPOR, TGFbR, PGE2’s EP2R and EP4R, and LPS receptors, likely through TLR2 or TLR4. Among these receptors, the signal transduction mechanisms employed by TCR to activate HPK1 are the most comprehensively understood. HPK1 is now considered a valuable target in the fields of immuno-oncology and medicinal chemistry due to its pivotal role in immune cell activation and its potential to enhance the cancer surveillance capabilities of the immune system.
Downloads
Article in PDF
Recent Articles
- ASH 2022: Roche’s Polivy vs ADC Therapeutics’ Zynlonta, Antibody-Drug Conjugates (ADC) in R/R DLBCL
- Vivos Therapeutics ‘s DNA Oral Appliance for the Treatment of OSA; Arthrex’s TightRope Implant fo...
- FDA Approval to DePuy TELIGEN System; FDA Breakthrough Device Designation for the EndoStim System...
- Cancer Diagnostic Market: Evaluating the Major Growth Factors and the Key Developments in the Domain
- Potential of EGFR Inhibitors: A Promising Avenue in Cancer Treatment
The Potential of HPK1 Inhibitors in Immuno-Oncology
In the realm of immuno-oncology therapy for cancers, the inhibition of HPK1 emerges as a focal point, applicable to both responsive and non-responsive cases to current Immune Checkpoint Blockers (ICBs). HPK1 functions by downregulating the ERK/MAPK pathway, consequently influencing TCR-triggered IL-2 gene transcription, as well as fostering T cell activation and proliferation.
In recent years, there has been a growing emphasis on the research and development of HPK1 inhibitors. Hematopoietic progenitor kinase 1 has emerged as a highly regarded target for T cell-based immunotherapies. As a member of the serine/threonine MAP4K family, HPK1 is predominantly expressed in hematopoietic cell lineages and functions as a negative regulator of the T cell receptor (TCR) signaling pathway. Additionally, accumulating evidence indicates that the kinase activity of HPK1 inhibits immune functions not only in T cells but also in various other immune cell subsets such as B cells and dendritic cells. These findings collectively underscore the potential of small-molecule HPK1 inhibitors as an appealing approach in cancer immunotherapy, whether used as standalone agents or in combination with immune checkpoint inhibitors.
Promising HPK1 Inhibitors on the Horizon
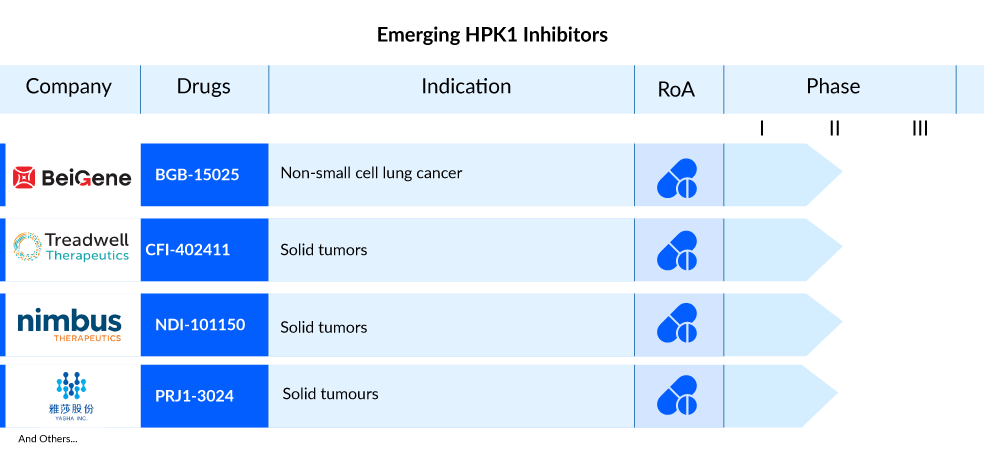
HPK1 holds significant therapeutic promise in immuno-oncology due to its involvement in various elements of the adaptive immune system, including T cell, B cell, and dendritic cell-mediated immune responses. The field of HPK1 inhibitors for cancer treatment is robust, with notable contributors like BeiGene (BGB-15025), Treadwell Therapeutics (CFI-402411), Zhuhai Yufan Biotechnologies (PRJ1-3024), Nimbus Therapeutics (NDI-101150), and other key players actively engaged in advancing promising products.
BGB-15025, developed by BeiGene, is a novel inhibitor targeting hematopoietic progenitor kinase 1 (HPK1) and is currently under investigation. HPK1 functions as a crucial negative feedback regulator in T-cell receptor signaling, a process thought to be integral to the body’s immune response against tumors. Preclinical studies have demonstrated that inhibiting HPK1 enhances T-cell activation, thereby potentially augmenting the effectiveness of anti-PD-1 inhibitors like BeiGene’s tislelizumab in combating tumors. BGB-15025 is presently undergoing Phase I trials to evaluate its safety and tolerability, both as a standalone treatment and in combination with tislelizumab, for individuals with advanced solid tumors.
CFI-402411 by Treadwell Therapeutics is a highly effective inhibitor of HPK1, and preclinical investigations have demonstrated its ability to stimulate the immune system. This includes relieving the inhibition of T-cell receptors (TCR), disrupting abnormal cytokine expression, modifying the immunosuppressive environment related to effector cells such as Regulatory T cells (Treg), and exhibiting strong anti-leukemic effects in various mouse models. Presently, the drug is undergoing Phase I/II trials to evaluate its safety and tolerance in individuals with advanced solid malignancies, both as a standalone treatment and in combination with pembrolizumab.
NDI-101150 is a specific inhibitor of hematopoietic progenitor kinase 1 (HPK1). Nimbus Therapeutics reported the initiation of the first-in-human Phase I/II study in November 2021, with the first patient being dosed. At present, NDI-101150 is undergoing Phase I/II trials to assess its safety, tolerability, pharmacokinetics, and initial antitumor effects when administered alone or in conjunction with pembrolizumab for patients with solid tumors.
PRJ1-3024 (Zhuhai Yufan Biotechnologies), an oral HPK1 inhibitor, is being tested in a Phase I trial to assess its safety and initial effectiveness in individuals with relapsed or refractory solid tumors. It’s administered once daily for this investigation.
Expected Roadblocks in the HPK1 Inhibitors Market
The HPK1 inhibitors market faces several barriers that impact its growth and adoption. Firstly, a significant challenge lies in the complex and dynamic nature of the biological systems these inhibitors target. The intricacies of signaling pathways involving HPK1 necessitate a thorough understanding, making drug development and testing a time-consuming and resource-intensive process. Moreover, the potential for off-target effects poses a substantial obstacle. Ensuring the specificity of HPK1 inhibitors is crucial to avoid unintended consequences on other cellular processes.
Regulatory hurdles further impede progress. The stringent requirements set by health authorities demand comprehensive preclinical and clinical data, adding both time and cost to the development timeline. Additionally, the limited understanding of the long-term effects and potential side effects of HPK1 inhibition raises concerns, both among regulatory bodies and healthcare providers.
Market competition is another barrier. The pharmaceutical industry is highly competitive, and the presence of alternative therapeutic approaches or drugs addressing the same indications can divert attention and investment away from HPK1 inhibitors. This makes it crucial for companies in this market to demonstrate a clear clinical advantage and cost-effectiveness over existing or emerging treatments.
Furthermore, reimbursement challenges and pricing pressures can hinder market access. Payers may be reluctant to cover the costs of novel and potentially high-priced HPK1 inhibitors without robust evidence of their efficacy, safety, and economic value. Overcoming these barriers requires a collaborative effort from researchers, regulatory agencies, and industry stakeholders to advance the development, approval, and market integration of HPK1 inhibitors.
The Unfolding Story of HPK1 Inhibitors: What Lies Ahead
HPK1 inhibitors represent a promising frontier in the field of drug development, with a bright future outlook. As key players in immune regulation, HPK1 inhibitors hold immense potential for treating a variety of immune-related disorders, including autoimmune diseases and certain cancers. The inhibition of HPK1 can modulate signaling pathways that regulate immune responses, making it an attractive target for therapeutic interventions. Researchers are actively exploring the potential of HPK1 inhibitors in preclinical and clinical studies, with early results demonstrating their efficacy in dampening excessive immune activation. The future may see the development of novel HPK1 inhibitors with improved specificity, bioavailability, and reduced side effects, further enhancing their therapeutic profile. As our understanding of the intricate immune system continues to evolve, HPK1 inhibitors are poised to play a pivotal role in the next generation of immunomodulatory therapies, offering new hope for patients facing challenging medical conditions. Continued research and development in this field are likely to unlock the full therapeutic potential of HPK1 inhibitors, ushering in a new era of precision medicine for immune-related disorders.

Downloads
Article in PDF
Recent Articles
- Top 10 Expected Oncology Drug Launches in 2023
- Antibody-Drug Conjugate: The Smart Biological Bomb
- BGB-16673 Breakthrough: A Tolerable Triumph in Rapid Clinical Responses for Relapsed/Refractory B...
- How is Nanomedicine Transforming the Dynamics of the Healthcare Industry?
- Vivos Therapeutics ‘s DNA Oral Appliance for the Treatment of OSA; Arthrex’s TightRope Implant fo...