Novel Insights Into New Therapies for HR+/HER2- Breast Cancer
Aug 08, 2024
Table of Contents
Breast cancer is a serious and most common problem in developed as well as developing countries.
According to the World Health Organization (WHO), more than 2.3 million cases of breast cancer occur each year, making it the most common cancer among adults. In 95% of countries, breast cancer is either the leading or second-leading cause of female cancer deaths. However, survival rates for breast cancer are widely inequitable, with nearly 80% of deaths from breast and cervical cancer occurring in low- and middle-income countries.
Downloads
Article in PDF
Recent Articles
- Össur Launches POWER KNEE; DePuy Synthes Acquires CrossRoads Extremity Systems; Myomo’s MyoPro; A...
- Top 5 Cancers Creating Major Challenge To The Global Healthcare System
- Ypsomed-CamDiab’s Collaboration; Vela Diagnostics’s NGS-Based Pan-Cancer Panels; Fresenius&...
- Intellia’s Quest with CRISPR; Innovent, Synaffix ADC Tech Deal; Lilly’s Diabetes Bloc...
- Notizia
Additionally, according to Delveisnight, breast cancer is the most common cancer in women in the United States, excluding skin cancers, accounting for about 30% (or 1 in 3) of all new female cancers each year. Approximately 0.5–1% of breast cancers occur in men. The HR+/HER2- subtype is the most common, with an age-adjusted rate of 87.2 new cases per 100,000 women in the United States. Postmenopausal women account for 80–90% of breast cancer cases, while premenopausal women represent a significantly smaller proportion.
What are the Breast Cancer Survival Rates?
Breast cancer is the second-most common cause of death in women after lung cancer. However, the number of women dying due to it has lowered down gradually owing to improved diagnosis and treatment methods. As per the analysis, ~64% of breast cancer patients have local-stage breast cancer, 27% have regional stage, and 6% have distant (metastatic) disease.
Different sub-types of Breast cancer affect people differently. Some behave aggressively on some compared to others, some tend to prevail due to fewer feasible treatment options. Other factors, such as if cancer has spread to other parts of the body like bones, i.e., the extent and location of the metastasis, affect the long-term survival of the patients. Among the various disease subtypes, ER+/HER2- has the highest number of cases, ∼140K in 2022 and 200K in 2023, followed by Triple-negative with around ∼24K cases and ER+/HER2+ with about ∼20K cases. HR-/HER2+ has the least number of cases.

Immediate treatment and the effectiveness of the therapy can also improve the survival rates.
According to a survey by Cancer.net, the average 5-year survival rate for women with invasive breast cancer is 90% I.e., 90% of women living with invasive breast cancer will survive for at least 5 years. Whereas the per cent for 10-year survival rate falls to 83%.
Furthermore, the 5-year survival rate in case of metastatic cancer falls depending upon the part cancer has invaded.
Know Your Type of Breast Cancer
Knowing the type of breast cancer is as important as knowing the disorder a woman is suffering from. The genetic makeup of the tumor helps physicians detect and choose the right approach to treat cancer.
The status of hormone receptor (HR) and human epidermal growth factor receptor- 2 (HER-2) defines the type of breast cancer. These two receptors can either be present or absent. According to the estimates, most cases of HR+/HER2− breast cancer occur in people aged between 60 to 79 years in the United States, which were nearly 100,000 cases in 2023.
The stage of breast cancer plays a crucial role in determining the best treatment approach. For women diagnosed with Stage I, II, or III breast cancer, treatment typically begins with surgery, often followed by radiation therapy. Additionally, drug therapy is a common part of the treatment plan for these stages. Depending on the tumor’s characteristics, treatments may include chemotherapy, hormone therapy (such as tamoxifen or an aromatase inhibitor), HER2-targeted drugs like trastuzumab (Herceptin) and pertuzumab (Perjeta), or a combination of these therapies.
Stage I Breast Cancer Treatment
For Stage I breast cancer, hormone therapy is frequently recommended for women with HR+ (ER+ or PR+) tumors, regardless of the tumor’s size. This treatment can involve tamoxifen, an aromatase inhibitor, or a sequence of both. Women with tumors larger than 0.5 cm are more likely to benefit from hormone therapy, which is typically administered for at least 5 years. If the tumor exceeds 1 cm, adjuvant chemotherapy may also be considered, particularly if the cancer exhibits aggressive features like fast growth, HR-, or HER2+ status, or a high gene panel score. For HER2+ tumors, adjuvant trastuzumab is usually recommended for 6 months to a year.
Stage II Breast Cancer Treatment
Systemic therapy is a cornerstone for Stage II HR+ HER2-negative breast cancer treatment. This can include neoadjuvant therapy, administered before surgery, or adjuvant therapy, given after surgery. Neoadjuvant therapies are especially beneficial for women with large tumors, as they can shrink the tumor sufficiently to make breast-conserving surgery a viable option. However, this approach does not necessarily improve survival rates compared to adjuvant therapy. In some cases, systemic therapy starts before surgery and continues afterward.
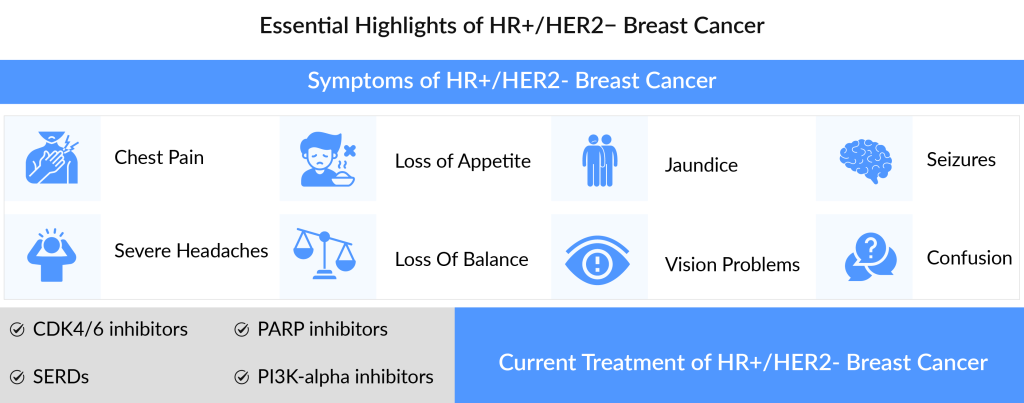
Stage III Breast Cancer Treatment
Stage III cancers often require neoadjuvant chemotherapy before surgery. For HER2+ tumors, targeted therapies like trastuzumab, sometimes combined with pertuzumab, are used to shrink the tumor and possibly enable breast-conserving surgery. If the tumor does not shrink enough, a mastectomy may be necessary. In Stage III cases, nearby lymph nodes are also assessed, often requiring an axillary lymph node dissection (ALND) instead of a sentinel lymph node biopsy (SLNB).
HR+/HER2- Breast Cancer Market Outlook
The HR+/HER2- breast cancer market size in the 7MM was observed to be USD 7.4 billion in 2022 and 7.5 billion in 2023.
In 2022, the market size of ER+/HER2- breast cancer in first-line setting in the United States was around USD 3.8 billion.
In 2023, the market size for HR+/HER2− breast cancer in the EU4 and the UK was approximately USD 2.3 billion.
Of all the prescribed first-line therapies, CDK4/6 inhibitors market occupy the maximum market share, Ibrance leading the charts, followed by Kisqali and Verzenio. Anticipated new therapies include Lasofoxifene, Camizestrant, Capivasertib, ARV-471, and others. These upcoming combination therapies are expected to enhance the current market by providing more effective treatments with fewer adverse effects.
In 2023, IBRANCE led the market with the largest revenue, generating around USD 4.5 billion.
Among the newer therapies, CDK4/6 inhibitors—such as palbociclib, ribociclib, and abemaciclib—are gaining significant attention. These drugs target the cyclin D1-CDK4/6 complex, crucial for cell cycle progression. Approximately 29% and 14% of HR+/HER2-negative breast cancer patients have cyclin D1 and CDK4 amplification, respectively. Even when tumors develop resistance to hormonal treatments, they still rely on the CDK4/6-cyclin D1 pathway for growth. Consequently, combining hormonal therapy with CDK4/6 inhibitors leads to a more pronounced G1-S cell cycle arrest. These inhibitors work by blocking the phosphorylation of retinoblastoma protein, thus downregulating E2F-response genes and inhibiting cell proliferation.
Top Companies Transforming the HR+/HER2− Breast Cancer Landscape
The breast cancer market is poised for significant changes in the coming years due to the growing interest of various companies in developing targeted drugs for HR+/HER2−negative breast cancer. Increased awareness and the expanding use of approved breast cancer drugs are driving this shift. Leading players in this space include Olema Pharmaceuticals, Arvinas, Immutep, AstraZeneca, Roche/Genentech, Evexta Bio, EQRx, Sermonix Pharmaceuticals, Veru Pharma, Evgen Pharma, Eli Lilly, and others.
Recent Updates and Drug Developments
- In April 2024, Evexta Bio announced an update on the clinical development of rupitasertib. The Phase II trial is scheduled to begin enrolling patients in the fourth quarter of 2024. This study will assess the safety and efficacy of rupitasertib in combination with elacestrant for patients with ESR1 mutation ER+ HER2−negative advanced breast cancer.
- Additionally, in April 2024, the Biologics License Application for Datopotamab deruxtecan was accepted in the US for patients with previously treated metastatic HR+/HER2−negative breast cancer. The regulatory decision under the Prescription Drug User Fee Act is anticipated in the first quarter of 2025.
Emerging HR+/HER2− Breast Cancer Therapies and Drugs
Several emerging therapies are making waves in the treatment of HR+/HER2−negative breast cancer:
- VERZENIO (abemaciclib), developed by Eli Lilly, is a CDK4/6 inhibitor that has received breakthrough therapy designation from the US FDA for patients with HR+/HER2− advanced or metastatic breast cancer.
- IBRANCE (palbociclib), developed by Pfizer, is a kinase inhibitor approved by the FDA in the US, the EMA in Europe, and the PMDA in Japan. It is used in combination with an aromatase inhibitor for treating HR+/HER2− advanced or metastatic breast cancer.
- KISQALI (ribociclib), developed by Novartis, is another CDK4/6 inhibitor that targets cyclin-dependent kinases, crucial for cell cycle progression and cellular proliferation.
- PIQRAY (alpeslib), developed by Novartis, is an oral alpha-specific PI3K inhibitor that has shown potential in inhibiting the PI3K pathway and exhibiting antiproliferative effects in breast cancer cell lines with PIK3CA mutations.
- ORSERDU (elacestrant), developed by Stemline Therapeutics (Menarini Group), functions as a selective estrogen receptor degrader (SERD). It binds to estrogen receptor-alpha (ERα), blocking its transcriptional activity and promoting its degradation.
These advancements highlight the ongoing innovation in breast cancer therapies, promising more effective treatment options for patients battling this challenging disease.
Conclusion
In conclusion, the future of breast cancer treatment is becoming increasingly promising with the continuous advancements in breast cancer drugs and breast cancer medication. As the HR+/HER2− breast cancer incidence continues to rise globally, the market for innovative breast cancer therapies is expanding, showcasing the need for more effective and targeted solutions. Breast cancer surgery remains a critical component of treatment, but the integration of new drugs and emerging therapies is transforming patient care.
Recent developments, including novel therapies and potential breast cancer vaccines, highlight the commitment to improving patient outcomes. The ongoing research into new HR+/HER2−negative breast cancer medications and the dynamic nature of the HR+/HER2−negative breast cancer market emphasize the importance of personalized approaches to manage various subtypes of the disease effectively.As we advance, the evolving landscape of HR+/HER2−negativebreast cancer treatment promises to enhance survival rates and offer more effective options for patients. The combination of innovative drugs, advanced therapy for breast cancer, and strategic surgical interventions marks a hopeful and forward-looking approach in the fight against breast cancer.

Downloads
Article in PDF
Recent Articles
- Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy
- Eli Lilly’s Jaypirca Approval; Novartis’ Adakveo EMA Review; Janssen’s CARTITUDE-4 Study of CARVY...
- Now, Nanoparticles for Cancer Treatment
- Össur Launches POWER KNEE; DePuy Synthes Acquires CrossRoads Extremity Systems; Myomo’s MyoPro; A...
- Triple Negative Breast Cancer Responds to TINAGL Therapy