IgA Nephropathy – Navigating the Emerging Therapies and Key Companies in the Therapeutics Domain
Nov 05, 2021
Table of Contents
IgA Nephropathy (IgAN), also called Berger’s disease, is an autoimmune rare disease affecting the kidneys. It impairs blood filtration in the kidneys’ tiny blood channels. An aberrant protein destroys the filtering unit (glomerulus) inside the kidneys, causing IgA Nephropathy. It is estimated that 20–40% of persons with IgAN would develop end-stage kidney disease, necessitating dialysis or kidney transplantation. It can present in various ways, ranging from asymptomatic microscopic hematuria to quickly progressing GN. Depending on the age group and biopsy practice patterns, the typical mode of presentation differs. Hypertension, proteinuria >1.0 g/day, male gender, reduced glomerular filtration rate (GFR) and severe pathologic findings on initial renal biopsy, a family history of chronic nephritis, susceptibility to the common cold, preference for salty foods, frequent consumption of raw eggs, and a high carbohydrate intake are all risk factors linked to progressive IgAN. Genetic factors also play a key role in the development and progression of IgA Nephropathy disease. It has been proposed that IgA Nephropathy is a complex polygenic disease, which means that many genes and environmental factors contribute to an individual developing the condition.

Moreover, in the early stages, the individuals do not experience any IgA Nephropathy symptoms, and as a result, the disease goes undetected for years or decades. When symptoms do appear, the most common IgAN symptom is hematuria or blood in the urine. The other IgA Nephropathy symptoms that IgAN patients often present with are:
Downloads
Article in PDF
Recent Articles
- Travere’s FILSPARI Approval Sparks Rivalry in the IgA Nephropathy Space
- Novartis’ FABHALTA: Leading the Way as the First Complement Inhibitor for IgAN
- Merck’s WINREVAIR™ EU Approval; BALVERSA for Urothelial Carcinoma; Novartis and Versant’s Boreali...
- Sanofi to Acquire Provention Bio; USFDA Committee Votes in Favor Roche’s Polivy; FDA to Rev...
- AbbVie Presents Phase III CANOVA Study Results; Novartis’ Iptacopan Shows Promise in Phase III St...
- Pain on the sides of the back (flank pain)
- Swelling in the ankles
- High blood pressure
But some patients with either rapidly progressive IgA Nephropathy or chronic asymptomatic disease may present with end-stage renal disease symptoms such as:
- Little or no urination
- Oedema
- Feeling tired
- Drowsiness
- Generalised itching or numbness
- Dry skin
- Headache
- Weight loss
This condition is most common in Caucasian and Asian males. It usually manifests itself in people in their twenties to late thirties, but it can occur at any age.
IgA Nephropathy Epidemiology
IgA Nephropathy is a common glomerular disease across the world, but its prevalence varies greatly by geography. Several studies have been conducted to assess IgA Nephropathy prevalence in various global regions and investigate the factors contributing to geographic differences. As per DelveInsight’s IgA Nephropathy Market Report, the total IgA Nephropathy prevalence was 377, 829 in the 7MM in 2020, with the US accounting for the maximum prevalence. Among the EU5 countries, Germany had the highest IgA Nephropathy prevalent cases in 2020.
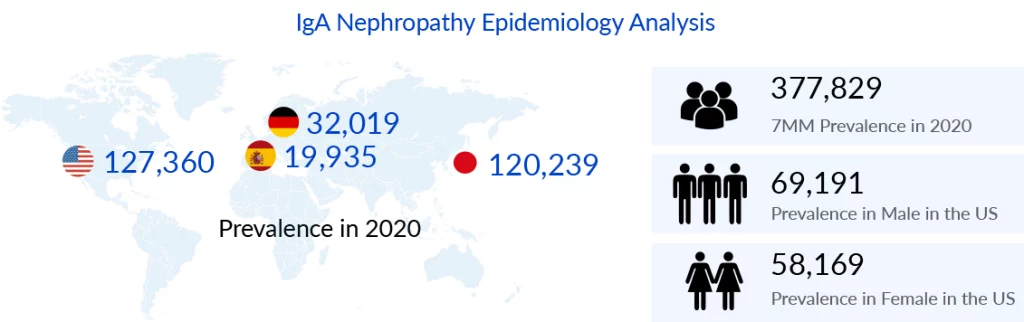
Another recently published report on IgA Nephropathy Epidemiology indicates that Males are more likely to be affected than females by primary IgAN. In 2020, the United States had 69,191 males and 58,169 females with IgA Nephropathy. IgAN is more common in young adults (>45 years old), although it can also be found in children and teenagers with isolated microscopic hematuria (up to 35%) or hematuria combined with non-nephrotic proteinuria (30%).
Globally, IgA nephropathy is the most frequent primary glomerulonephritis that can lead to renal failure. Although the specific pathophysiology of IgAN is still unknown, recent biochemical and genetic evidence points to the overproduction of abnormally glycosylated IgA1.
IgA Nephropathy Diagnosis and Role of Oxford Classification in Prognosis
The gold standard for IgAN diagnosis is kidney biopsy revealing IgA as the dominant or co-dominant immunoglobulin in the glomerular mesangium. The pathophysiology of IgAN, on the other hand, is unknown. Although kidney biopsy is still the only method for IgAN diagnosis, recent research has focused on finding novel biomarkers that could potentially predict IgAN without requiring a risky, invasive biopsy.
The Oxford classification was created in 2009 as a pathological categorization system for IgA Nephropathy to predict disease progression risk. The Oxford Classification of IgA Nephropathy (IgAN) is the most extensively used approach for evaluating IgA Nephropathy histologic features. Only three reproducible characteristics identified on the renal biopsy in IgAN independently predicted the outcome. According to the classification, they gave prognostic information and prognosis prediction based solely on clinical aspects. Mesangial hypercellularity (M), segmental glomerulosclerosis (S), and tubular atrophy/interstitial fibrosis (T) are the three characteristics. Furthermore, patients with endocapillary hypercellularity (E) who received immunosuppressive medication had a considerably lower rate of renal functional deterioration. These four factors, known as MEST scores, are included in the Oxford Classification.
Furthermore, disease associations and other organised programs for IgAN patients are established in most of the regions. Some prominent ones include IGA Nephropathy Foundation, National Kidney Foundation, Nephcure Kidney International, and others. All of these provide opportunities to map IgAN patients and improve their access to innovative treatments and outcomes. The establishment of country-wide registries in co-operation with these centres, in the near future, may also help in tackling the disease further.
IgA Nephropathy Treatment Market
There is currently no disease-specific IgA Nephropathy treatment. Medications that help manage symptoms like high blood pressure, protein in the urine, and swelling, as well as decrease the progression of the disease, are used as IgAN treatment. Though the condition’s exact cause is unknown, many different forms of immunosuppressants, or immune-suppressing medicines, such as steroids, are also considered. Angiotensin-converting enzyme inhibitors (ACE inhibitors) and angiotensin-receptor blockers (ARBs) are two of the most common IgA Nephropathy treatments.
According to DelveInsight’s IgA Nephropathy market report, the IgA Nephropathy treatment market size was USD 109.3 million in 2020, with the US accounting for the maximum share (66.79%) of the overall market.

The future of the IgA Nephropathy market is optimistic with emerging IgAN therapies such as Nefecon (Calliditas Therapeutics), Sparsentan (Travere Therapeutics), Iptacopan (Novartis Pharmaceuticals), Narsoplimab (Omeros), Atrasentan (Chinook Therapeutics Inc.), among others.
With a plethora of drugs in the IgAN pipeline, the market size is projected to increase. Among the emerging IgA Nephropathy therapies, the most promising ones include Narsoplimab- a Phase III Monoclonal antibody, Iptacopan – a Phase III Small molecule, Nefecon – a Phase III Glucocorticoid receptor agonist, among others.
| Drug | Phase | MoA | Route of Administration | Company |
| Nefecon | Phase III | Glucocorticoid receptor agonist | Oral | Calliditas Therapeutics |
| Sparsentan | Phase III | Angiotensin type 1 receptor antagonists | Oral | Travere Therapeutics |
| Iptacopan | Phase III | Small Molecule inhibitor of factor B | Oral | Novartis |
| Narsoplimab | Phase III | MASP2 protein inhibitors | Intravenous/Subcutaneous | Omeros Corporation |
| Atrasentan | Phase III | Endothelin A receptor antagonists | Oral | Chinook Therapeutics |
Expected Roadblocks
IgAN affects a small, heterogeneous, and widely distributed patient group, complicating study enrollment, design, and replication in clinical trials. IgAN is difficult to diagnose because of its nature. During this process, valuable time is squandered, delaying access to novel treatments and resulting in substantial losses. Measuring IgAN clinical trial outcomes in patients is difficult because their clinical presentations and histories are typically highly diverse. Whether they are clinician-reported, observer-reported or patient-reported outcomes, variables such as age, disease progression, and disease severity influence reported outcomes (PROs). Robust trials have traditionally been hindered by low recruitment, given the relative rarity of IgA Nephropathy, and there has been a scarcity of globally recruiting clinical research trials.
IgA prevalence varies significantly among continents and ethnic groups. It is reported to be highest in industrialised Asian countries such as Japan (31%), followed by several European countries (20–30%) and the United States (10–20%). Different healthcare screening strategies, biopsy techniques, and genetic and environmental factors could contribute to this variation. The wide range of prevalence and course of IgAN makes it challenging to develop consistent, evidence-based treatment options.
In East Asian countries like Japan and South Korea, annual urine analysis in schools and workplaces is legally mandated. In Japan, health screening was improved from biannual to annual in 1979. In Japanese individuals aged 40 years and older, a combination of urinalysis and serum creatinine level assessment has helped to detect early-stage renal disease. The Japanese kidney disease screening program has been beneficial in detecting milder forms of glomerulonephritis, especially endemic IgAN, allowing for early care and possibly delaying the need for renal replacement treatment.
However, there is no national kidney disease screening program in most Western countries, such as the United States, the United Kingdom, and others. As a result, recognising small symptoms like proteinuria that warrant a kidney biopsy depends on various circumstances such as health insurance market access, socioeconomic considerations, and so on. There are also discrepancies in physician procedures when it comes to ordering a kidney biopsy. These factors add up to delayed IgAN diagnosis, misdiagnosed patients, and even undiagnosed instances.
Way Ahead
IgA Nephropathy is still a common cause of glomerular disease and contributes significantly to the global burden of Chronic Kidney Diseases and End-Stage Renal Disease. However, the IgA Nephropathy market is experiencing a robust pipeline with novel therapeutic targets, increased scientific advances, and the identification of novel biomarkers for improved diagnosis and disease progression monitoring. According to the DelveInsight estimates, the IgAN treatment landscape looks promising with greater advances in diagnosis and treatments that translate into a better quality of life and improved survival. The entry of the novel therapies necessitates collaboration among academia, life-sciences companies, funding, regulatory agencies, decision-makers, and caregivers to attain better healthcare. Furthermore, it is hoped that other putative therapeutic agents may emerge as a result of ongoing research, which will alter the IgA Nephropathy treatment landscape in the near future.
IgA Nephropathy is a rare, autoimmune disease affecting the kidneys. It impairs blood filtration in the kidneys’ tiny blood channels. An aberrant protein destroys the filtering unit (glomerulus) inside the kidneys, causing IgA Nephropathy.
There is currently no disease-specific IgA Nephropathy treatment. But certain medications can slow the progression of the disease. Many different forms of immunosuppressants or immune-suppressing medicines, such as steroids, along with Angiotensin-converting enzyme inhibitors (ACE inhibitors) and angiotensin-receptor blockers (ARBs) are used for IgA Nephropathy treatment.
In the early stages, the individuals do not experience any IgA Nephropathy symptoms, and as a result, the disease goes undetected for years or decades. When symptoms do appear, the most common IgAN symptom is hematuria or blood in the urine. The other IgA Nephropathy symptoms that IgAN patients often present with are:
Pain on the sides of the back (flank pain)
Swelling in the ankles
High blood pressure
But some patients with either rapidly progressive IgA Nephropathy or chronic asymptomatic disease may present with end-stage renal disease symptoms
The leading companies involved in developing novel therapies for IgAN treatment include Calliditas Therapeutics, Travere Therapeutics, Novartis Pharmaceuticals, Omeros, Chinook Therapeutics Inc., and others.
The future of the IgA Nephropathy market is optimistic with emerging IgAN therapies such as Nefecon (Calliditas Therapeutics), Sparsentan (Travere Therapeutics), Iptacopan (Novartis Pharmaceuticals), Narsoplimab (Omeros), Atrasentan (Chinook Therapeutics Inc.), among others.
Downloads
Article in PDF
Recent Articles
- Biogen’s SPINRAZA Phase II/III Trial Results; Travere’s FILSPARI FDA Approval; GSK’s ...
- 4 Late-Stage IgA Nephropathy Treatment Drugs to Look Out
- Travere’s FILSPARI Approval Sparks Rivalry in the IgA Nephropathy Space
- Recent Inclination of Pharma Companies, Emerging Pipeline Therapies Drive the IgA Nephropathy Mar...
- Roche’s Columvi Phase III STARGLO Trial; Novartis’ Fabhalta Latest Data; Vertex’s Alpine Im...