Widespread Usage of HPV Vaccine Reduces Cervical Cancers and Precancers
Nov 08, 2024
Table of Contents
Human papillomavirus (HPV) is a very commonly found virus, and most people have experienced it at some point in their lives. There are almost 200 different strains that can be split into low-risk and high-risk. Low-risk HPV usually doesn’t cause many problems, it usually causes conditions like genital warts. Whereas High-risk HPV at times can cause serious problems such as Cervical Cancers. In women, HPV-related cancer, especially Cervical Cancer, is considered to be the most common cancer. Almost all cervical cancers are caused by Human papillomavirus.
Women will always remain at risk of HPV infection throughout their lives, which mostly includes cervical cancer. Cervical cancer is caused by HPV types 16 and 18. Approximately 100 types of HPV have been identified to date, and of these, approximately 15 virus types are known to cause cervical cancer. The HPV vaccine can help protect against many types of HPV infections that can cause cervical cancer.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Regeneron/Sanofi’s PD-1 drug candidate; Zika targeting drugs and vaccines developments; CMO of Ac...
- Cervical Cancer Market Has A Lot Going On Under The Hood
- C4X Discovery and AstraZeneca Signs Deal; FDA Rejects Spectrum’s Poziotinib; Orphan Drug Designat...
- Merck, Gilead teams up for HIV treatment; Bright Future for Sanofi/Regeneron’s Libtayo; Roche acq...
- Subtypes of cervical cancer identified
Cervical cancer is considered to be the second leading cause of cancer death in women in their twenties and thirties. The HPV vaccine can help protect people from several types of HPV infections that can cause cervical cancer and other cancers as well, such as vulva, penis, vagina, anus, throat, and mouth cancers. The first-ever HPV vaccine protected against different HPV types i.e., 16 and 18. The vaccines that are used now help protect against almost 9 types of HPV, including types 16 and 18.
HPV Vaccination
The recommended age for HPV vaccine administration is between 9 and 12 years. Apart from that, children and young adults who haven’t been administered with the HPV vaccine or who haven’t gotten all their doses should be vaccinated soon. Vaccination provided at the recommended ages will be more beneficial and help prevent more cancers than vaccination at older ages.
There are three FDA-approved HPV vaccine types available to prevent infection: GARDASIL, GARDASIL 9, and CERVARIX. Since 2016, GARDASIL 9 has been the only HPV vaccine used mainly in the United States, while the other three are used commonly in the UK.
GARDASIL, an inactivated, quadrivalent HPV vaccine (types 6, 11, 16, 18), is currently manufactured by Merck Sharp & Dohme Corp. It provides protection against HPV types 16 and 18, two of the most high-risk HPVs that cause about 70% of cervical cancers. It also protects against two more types of HPV infection caused by types 6 and 11, which are responsible for 90% of genital warts. The FDA approved GARDASIL on June 8, 2006.
GARDASIL can be indicated to both males and females of 9 to 26 years of age. It can be used for protection against cervical, vulvar, vaginal, anal cancer, and genital warts in females whereas for protection against anal cancer and genital warts in males. GARDASIL should be administered intramuscularly as three, 0.5-mL doses completed within 6 months from the initial HPV vaccine doses.
Some of the side effects of the HPV vaccine include fever, nausea, headache, diarrhea, abdominal pain, and fainting. GARDASIL is pulled off from the US market since 2017 due to another more widely accepted vaccine from the same company.
CERVARIX – a bivalent (types 16 and 18), recombinant HPV vaccine is manufactured by GlaxoSmithKline. There are approximately 100 different types of HPV identified to date and CERVARIX does not protect against all of them. Rather, it is used only for prevention and protection against cervical pre-cancers and cervical cancer associated with oncogenic human papillomavirus (HPV) types 16 and 18 for young girls and women aged between 9 to 25 years.
GSK HPV vaccine CERVARIX is provided to young women between 10 to 25 years of age, as an HPV vaccine 3 dose schedule. The schedule must be completed within six months of the initial HPV vaccine doses. FDA approved CERVARIX on 16th October 2009. Till date, in around 100 countries, Cervix has already been approved, including 27 member states of the European Union (EU), Brazil, Australia, Mexico, Taiwan, and South Korea. However, in 2016, GSK voluntarily removed CERVARIX from the US market due to less demand and more consumption of GARDASIL 9 throughout the USA.
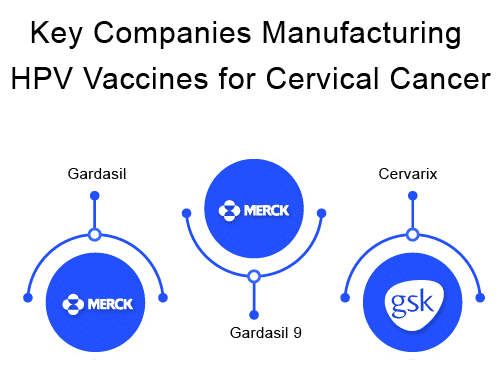
GARDASIL 9 – a 9 valent HPV vaccine prevents infection with the following nine HPV types – 6 and 11, types 16 and 18, and types 31, 33, 45, 52, and 58, which are considered high-risk HPVs that can cause cervical cancers. It is another Merck HPV vaccine. FDA approved GARDASIL 9 in December 2014 and is the most widely accepted HPV vaccine throughout the world because of its broad protection against 9 strains of HPV. In the USA, HPV vaccine has been the only continuous and available vaccine.
Initially, this vaccine was only approved for cervical cancer prevention, but in 2020 FDA approved it for the prevention of oropharyngeal cancer and other head and neck cancers, broadening its protection spectrum even more. Gardasil 9 is to be administered to both males and females from 9 to 45 years of age.
Patients from 9 to 14 years of age when receiving a 2 HPV vaccine doses, it should be given as 0, 6 – 12 months format, whereas if they are receiving a 3 HPV vaccine doses, it should be given as 0, 6, 12 months format. Apart from that patients aged 15 to 45 years are administered a three-dose HPV vaccine schedule that must be completed within six months of the initial HPV vaccine doses.
Read our blog Preventing Cervical Cancer Through Screening and HPV Vaccines for more insights
Salient Factors About the HPV Vaccine
The HPV vaccine provides protection against HPV that can cause different types of cancers, which mainly include cervical cancer, other cancers include anal cancer, vaginal cancer, and vulvar cancer. Some of the essential factors for HPV vaccine include-
- The very first HPV vaccine became available in the year 2006
- It is considered to be a part of routine recommended vaccination in every country
- These vaccines provide protection for almost 5 to 10 years
- HPV vaccines are observed to be very safe in nature, as for children who are mildly ill, people with low-grade fever (101 degrees), cold, cough, running nose, etc.
- Large population, even the unvaccinated get benefitted from the large portion of people who get vaccinated
- Mild side effects such as swelling, redness, and pain at injection sites are usually observed after administration
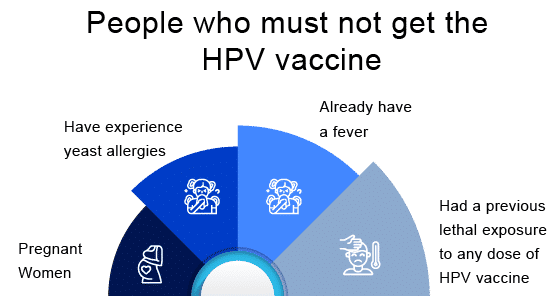
Now, some features for those people who must not get the HPV vaccine-
HPV Vaccine Mechanism of Action And Efficacy
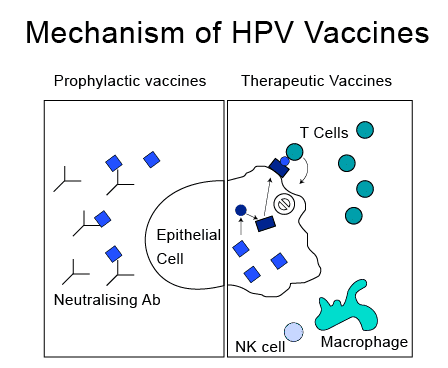
The HPV vaccine consists of individual viral-like proteins that copy (imitate) different types of HPV. These viruses are not live viruses, but they can still produce an immune response (antibodies) when administered into the body. Like other immunization processes, these HPV vaccines also generate antibodies in the body that would bind to the antigen of the virus, if the body gets infected in the future. HPV vaccination has been working exceptionally well in recent years. It is observed that the HPV vaccine immune response has the potential to protect against 90% of HPV-attributable cancers.
In the year 2006, the first HPV vaccination was recommended, the infection types with HPV, including cervical cancers and genital warts, have been seen to have dropped around 88% in teen girls and 81% among young adult women. Nowadays, due to the availability of HPV vaccines for teens and young adults, they are experiencing a reduction in genital warts.
Most notably, HPV vaccination has been the cause of a reduction in the number of precancer of the cervix (part of the uterus) cases in young women. The protection from this vaccine lasts for around 10 years and sometimes even more. When the people receiving HPV vaccines were followed up for at least 12 years of complete dosage, their protection remained substantially high with no evidence of decreasing over time.
Currently, the HPV vaccine is based on virus-like particles (VLPs) that form on the surface of HPV components. What makes virus-like particles (VLPs) non-infectious is the lack of the virus’s DNA. Nonetheless, they resemble the natural virus very closely, and antibodies against the VLPs also have activity against the natural virus. Through many studies and clinical trials, it is found that VLPs are strongly immunogenic, so they are able to induce very high levels of antibody production in the body.
The VLPs have been found to be strongly immunogenic, which means that they induce high levels of antibody production by the body. This makes these vaccines highly effective. All the HPV vaccines have been observed to have higher efficacy (even closer to 100%) for prevention from HPV infection. The initial trials that were conducted included women aged between 15 to age 26 years, which resulted in a three-dose HPV vaccine schedule.
After that, many immunogenicity trials conducted several years later led to the approval and recommendation of a two-dose HPV vaccine schedule in young adolescents. Ideally, females must get vaccinated before they become sexually active and there is a probability of getting exposed to HPV. These vaccines can not prevent other types of sexually transmitted diseases (STDs) or sexually transmitted infections, nor can they treat any existing HPV infections or HPV-caused disease. They can solely provide prior protection to human papillomavirus.
How Does HPV Vaccine Prevent Cervical Cancer
As it is known Human papillomavirus infection plays an important role in cervical cancer development. The HPV vaccine targets these particular cervical cancer-causing viruses, providing some protection against cervical cancer. HPV infections only occur in humans, affecting mucous membrane cells and the skin. They can be spread through direct contact with infected skin or mucous membranes.
HPV infections usually go unnoticed, don’t cause any symptoms, and clear up on their own. In rare cases, though, they gradually lead to the development of cervical cancer over many years or decades. In addition to the vaccine administration, regular screening can help in cervical cancer prevention upto a good extent. During the cervical cancer screening, abnormal cells (dysplasia) can be discovered and if necessary also removed. Pap tests are the most commonly used routine screening procedure for cervical cancer.
Infection with the HPV virus can cause the cells to alter in a way that they develop into cancer in the future, these HPV vaccines provide specific protection against particular types of HPV virus that cause cervical cancer. They are observed to reduce the number of abnormal cervical cells in women that have changed a lot and it is less likely that they might develop into cancer. It can be concluded that HPV vaccines provide very long-term protection against cervical cancer.
Possible Side Effects of HPV Vaccine
Just like any common drug or medicine, vaccines can also create significant side effects. It depends on each individual differently, many people who get HPV shots might get side effects whereas some might have no HPV vaccine side effects at all. Some people are reported to have very mild side effects, whereas some experience HPV vaccines long-term side effects. The most common mild HPV vaccine side effects are –
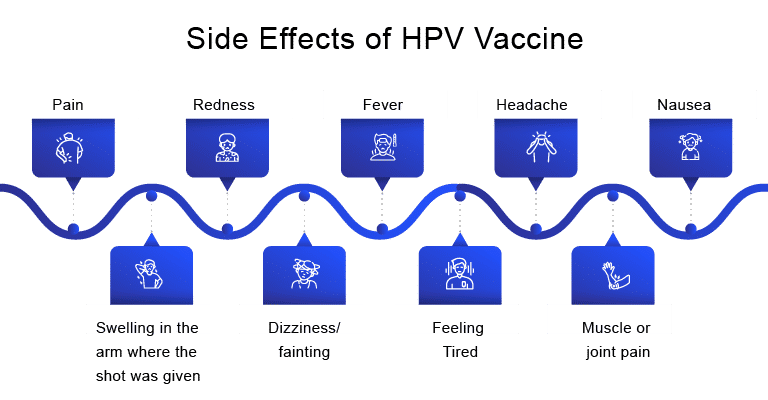
Very rarely, severe (anaphylactic) allergic reactions are observed after HPV vaccination. People with these types of severe allergies to any component of the HPV vaccine should immediately stop the administration. Some of the more serious HPV vaccine side effects include hives, difficulty in breathing, fast heartbeat, swelling of face and throat.
What Lies Ahead For HPV Vaccination
As per studies, cervical cancer is the second most common cancer among women worldwide. The efficacy and effectiveness of the quadrivalent, as well as the 9 valent HPV vaccine, is currently doing wonders in preventing high-grade cervical lesions as well as providing immunity to advanced cervical cancer. While current prophylactic vaccines like GARDASIL and CERVARIX are effective in preventing HPV infections, they do not treat existing cancers. However, new therapeutic candidates are emerging with the potential to change the landscape. Nykode Therapeutics’ VB10.16, for instance, shows promise when combined with PD-1 inhibitors for treating cervical and head and neck cancers. Similarly, ISA Pharmaceuticals’ ISA101b is advancing through trials targeting HPV16+ cancers. These advancements generate optimism for enhanced treatment outcomes and indicate potential growth in the market.
It is also observed that the incidence of cervical precancer and cancer has been reduced exponentially when a large number of people in a community get vaccinated. It is estimated that almost 70% of cervical cancer would be prevented with proper HPV vaccination. Currently, more than 100 countries have begun using HPV vaccination as a regular routine of vaccine schedule to help WHO plans to get close to eliminating cervical cancer worldwide.

Downloads
Article in PDF
Recent Articles
- Cervical Cancer Treatment: Navigating the Changing Landscape
- Bayer’s AskBio Initiates Phase II GenePHIT Trial; FDA Approves Merck’s KEYTRUDA Plus Chemoradioth...
- Regeneron/Sanofi’s PD-1 drug candidate; Zika targeting drugs and vaccines developments; CMO of Ac...
- Insights into the Evolving Landscape of Antibody-Drug Conjugate (ADC) & the Key Companies in...
- Human Papillomavirus (HPV) Prophylaxis– Expected to see a steady growth from 2015-2025