Valneva’s Ixchiq: First-Ever Chikungunya Vaccine
Dec 08, 2023
Over the past 15 years, an estimated 5 million cases of chikungunya have surfaced globally. This virus poses an escalating menace, having affected over half of the world’s nations. Recent cases in Spain, Italy, and France underscore its widespread reach. Additionally, the virus has expanded to new geographical areas, contributing to an increase in the worldwide prevalence of the disease.
Originating in Tanzania in 1952, chikungunya remains elusive to treatment, posing a potentially fatal risk to infants. In adults, it triggers fever and joint pain, accompanied by symptoms like headaches, muscle pain, rash, and joint inflammation. These effects may endure for months or even years, as per the FDA. US travelers venturing into tropical regions, where chikungunya prevails, are likely to receive advisories regarding preventive measures.
The transmission of the chikungunya virus occurs mainly through the bite of mosquitoes carrying the infection. Typical symptoms of chikungunya encompass fever and joint pain, often accompanied by a rash, headache, and muscle discomfort. In certain cases, individuals might endure prolonged and severe joint pain lasting months or even years. Management typically involves rest, adequate hydration, and the use of over-the-counter medications to alleviate pain and fever.
Downloads
Article in PDF
Recent Articles
- Collaborate for TYG Oncology; GE Healthcare and Valneva collaboration; UPS to Acquire Marken
- CEPI Grants $41.3 Million to Valneva; Innovent Achieves Phase III Success for Mazdutide; GSK’s BL...
- FDA Grants Priority Review to Merck’s Application for KEYTRUDA Plus Padcev; Roche and Carmot Ther...
- FDA Approves ZURZUVAE for Postpartum Depression; Astellas Drug Acquired in $5.9B Deal Wins FDA Ap...
- CTEXLI Approved for Cerebrotendinous Xanthomatosis; SIGX1094 Wins Fast Track for Diffuse Gastric ...
Challenges Associated with the Development of the Chikungunya Vaccines
The development of a Chikungunya vaccine has faced several challenges, hindering its progress and availability. Some of these hurdles include:
Complexity of the Virus: The Chikungunya virus is an RNA virus with multiple strains. Developing a vaccine that offers broad protection against various strains of the virus is challenging due to its genetic diversity.
Lack of Long-Term Immunity: The natural immunity after a Chikungunya infection might not provide long-lasting protection, making it challenging to design a vaccine that induces durable immunity.
Limited Understanding of Immune Response: The exact immune response needed for protection against Chikungunya is not entirely understood. This makes it difficult to design a vaccine that elicits the appropriate and lasting immune response.
Cost and Resources: Developing vaccines is expensive and requires substantial resources. Chikungunya is often prevalent in resource-limited areas where the allocation of funds for vaccine development might be inadequate.
Clinical Trials: Conducting clinical trials for vaccines involves significant time and resources. Finding suitable candidates, ensuring safety, and proving efficacy in human trials are crucial but demanding phases in vaccine development.
Regulatory Hurdles: Meeting regulatory standards and gaining approval from health authorities require rigorous testing and documentation, adding to the time and cost of vaccine development.
Global Priorities and Market Demand: Chikungunya primarily affects tropical and subtropical regions. Vaccines for diseases impacting wealthier or more globally widespread populations might receive more attention and funding compared to those primarily affecting developing regions.
Vaccine Delivery and Distribution: Even if a vaccine is developed, ensuring its accessibility and distribution to remote or affected areas can be challenging due to infrastructural limitations and logistical issues.
Despite these challenges, efforts are ongoing to develop an effective Chikungunya vaccine. Several vaccine candidates have shown promise in preclinical and early clinical trials, and researchers continue to explore different vaccine platforms and strategies to overcome these obstacles and bring a safe and effective vaccine to the market.
Entry of First-ever Chikungunya Vaccine – Ixchiq
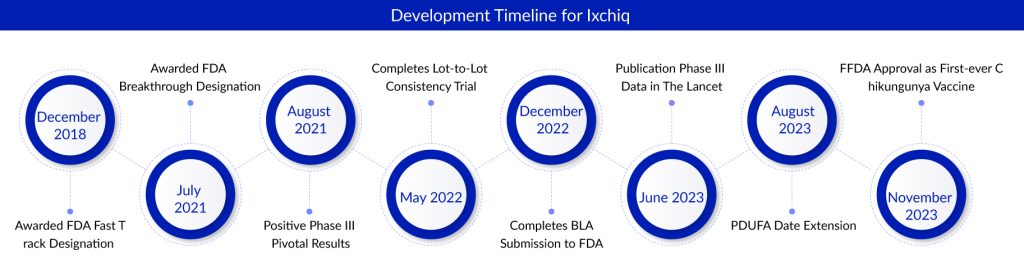
Valneva secured FDA approval for its chikungunya virus vaccine in the US, marking a significant milestone. Alongside the approval, the company also obtained a priority review voucher (PRV) that hastens drug application reviews from six to three months. Valneva plans to leverage this voucher by selling it, estimating an increase in revenue of approximately 90 million to 110 million euros ($96 million to $117 million) based on their 2023 earnings forecast. This news coincided with their quarterly earnings presentation, highlighting the strategic monetization of their R&D efforts.
Valneva emerged as the frontrunner in the competition to develop a chikungunya vaccine, surpassing several other contenders. In August, Bavarian Nordic unveiled promising outcomes from a phase III trial and is poised to introduce its Chikungunya vaccine in 2025. The Danish company acquired this vaccine, along with two others, from Emergent BioSolutions. Valneva’s single-dose, live-attenuated vaccine, named Ixchiq for commercial purposes, presents a viable option for adults facing an elevated risk of chikungunya virus exposure, particularly in tropical regions where mosquitos transmit the virus.
“We are committed to providing vaccines where there is a lack of medical solutions, aligning with our vision to play a role in a world free from the impact of preventable diseases through vaccination. Therefore, today signifies a significant stride in the prevention of chikungunya,” stated Valneva CEO Thomas Lingelbach.
The FDA’s accelerated approval was delayed by three months due to the necessity for additional time to finalize specifications for a phase 4 postmarketing confirmatory study. The approval was grounded on research demonstrating that 99% of recipients generated antibodies capable of neutralizing the virus in laboratory tests. Furthermore, monkeys infused with these antibodies contracted the virus but remained asymptomatic.
“Chikungunya virus infection can cause severe illness and long-term health issues, especially for older adults and those with pre-existing medical conditions. This approval marks a critical step in addressing an urgent medical gap and represents significant progress in preventing a potentially disabling disease with few available treatment choices,” remarked Peter Marks, head of the FDA’s Center for Biologics Evaluation and Research.
Valneva has outlined its strategy to launch Ixchiq in the US in the early months of the upcoming year. The Advisory Committee on Immunization Practices is scheduled to convene in late February to determine its recommendations for the vaccine.
“According to Juan Carlos Jaramillo, M.D., Chief Medical Officer at Valneva, over 75% of the global population resides in regions vulnerable to chikungunya transmission, a risk intensified by factors like climate change and global warming. With its presence in more than 110 countries, chikungunya stands as a prominent viral infection expected to expand into new geographical areas.”
Earlier this year, Merck halted the progress of its chikungunya vaccine candidate, obtained through the acquisition of Themis Bioscience for $366 million in 2020. Bharat Biotech in India is also actively engaged in the development of a chikungunya vaccine.
Advancing Toward a Resilient Future: Chikungunya Vaccine and Treatment Landscape
In recent years, promising advancements have been made in the development of Chikungunya vaccines. Several candidate vaccines, utilizing diverse platforms such as viral vectors, inactivated viruses, and mRNA technology, are undergoing clinical trials. These efforts aim to produce vaccines that not only provide robust immune responses but also ensure long-lasting protection against Chikungunya infection.
Moreover, beyond vaccines, the landscape for Chikungunya treatments is expanding with the exploration of antiviral drugs and immunomodulatory therapies. Researchers are investigating compounds that target key stages of the viral life cycle, offering potential avenues for the development of antiviral medications. Additionally, immunomodulatory approaches seek to mitigate the inflammatory response triggered by CHIKV infection, alleviating the severity of symptoms and preventing long-term joint damage.
While progress is being made, challenges such as vaccine distribution, access to treatment in resource-limited settings, and potential viral mutations remain on the horizon. Addressing these challenges requires a concerted effort to ensure equitable access to preventive and therapeutic interventions. Moreover, ongoing research efforts present opportunities to refine existing strategies and develop innovative solutions to combat Chikungunya and other arboviral threats.
In conclusion, the future Chikungunya vaccine and treatment landscape holds great promise, with advancements in research and development offering hope for a resilient defense against this persistent threat. As the scientific community continues to collaborate, innovate, and adapt to the evolving nature of infectious diseases, we move closer to a future where Chikungunya is no longer a major public health concern.

Downloads
Article in PDF
Recent Articles
- FDA Approves ZURZUVAE for Postpartum Depression; Astellas Drug Acquired in $5.9B Deal Wins FDA Ap...
- FDA Grants Priority Review to Merck’s Application for KEYTRUDA Plus Padcev; Roche and Carmot Ther...
- CTEXLI Approved for Cerebrotendinous Xanthomatosis; SIGX1094 Wins Fast Track for Diffuse Gastric ...
- CEPI Grants $41.3 Million to Valneva; Innovent Achieves Phase III Success for Mazdutide; GSK’s BL...
- Collaborate for TYG Oncology; GE Healthcare and Valneva collaboration; UPS to Acquire Marken



