7 Game-Changing Major Depressive Disorder Drugs in Development
Feb 28, 2025
Table of Contents
The Major Depressive Disorder drug treatment market is expanding rapidly due to the increasing demand for antidepressants and the disease’s profound impact on quality of life. In 2023, the major depressive disorder market across the 7MM was valued at an impressive USD 7.2 billion, and it’s poised for even greater expansion, with exciting growth on the horizon through 2034. The major depressive disorder treatment landscape has seen significant advancements, with multiple approved therapies playing a crucial role in symptom management. Currently, widely prescribed drugs for MDD include AbbVie and Gedeon Richter’s VRAYLAR, Axsome Therapeutics’ AUVELITY, Johnson & Johnson’s SPRAVATO, Otsuka’s REXULTI, and others. Regulatory agencies like the US FDA and the European Commission have accelerated approvals of innovative major depressive disorder therapies, further driving market growth.
VRAYLAR, marketed by AbbVie and Gedeon Richter, is one of the leading treatments. AUVELITY, an innovative oral therapy developed by Axsome Therapeutics, offers a fast-acting approach. Trintellix from Takeda, SPRAVATO from Johnson & Johnson, and REXULTI from Otsuka are also key therapies in the treatment landscape. Additionally, Fetzima from Forest Laboratories is another important option for patients.
Downloads
Article in PDF
Recent Articles
- Therapeutics and Celgene to develop therapies; Takeda’s expansion; J&J hopes to limit; Tech ...
- eCential Robotics’s Surgical Robotic Platform for Spine Surgery; Baxter Gets 510(k) Clearance for...
- Caristo Wins FDA Clearance for AI Solution to Prevent Heart Attacks; Lindus Health and Sooma Medi...
- Ipsen’s Cabometyx Rejected by NICE; Vertex and CRISPR Therapeutics’s Submit BLA to the FDA for ex...
- A Glance at Key Insights From 42nd J.P. Morgan Annual Healthcare Conclave
The market for major depressive disorder therapeutics in Europe is significant, with TRINTELLIX and SPRAVATO being widely prescribed treatments. In Jan 2025, the FDA approved SPRAVATO for use as a standalone treatment for adults with treatment-resistant depression. In Japan, approved therapies include TRINTELLIX and REXULTI, among others. Among various drug classes, Selective Serotonin Reuptake Inhibitors (SSRIs) dominate the major depressive disorder drugs market, given their long-standing effectiveness in managing depression. However, newer drug classes, such as Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) and Atypical Antipsychotics, are also making strides in improving treatment outcomes.
To learn more about SPRAVATO, dive into our latest blog crafted just for you!
7 Promising Major Depressive Disorder Drugs to Look Out
While current major depressive disorder treatments have improved patient outcomes, many individuals still struggle with treatment-resistant depression or experience unwanted side effects. To address these challenges, several promising drugs are in development, aiming to transform the major depressive disorder pipeline. Among the most anticipated therapies are COMPASS Pathways’ COMP360, SAGE Therapeutics and Biogen’s Zuranolone, Luye Pharma’s LY03005, Relmada Therapeutics’ REL-1017, Minerva Neurosciences and Janssen Pharmaceutical’s seltorexant, and BioLite and ABVC BioPharma’s ABV-1504, among others.
Let’s get into the details of these major depressive disorder drugs and explore their mechanisms of action, clinical progress, and potential impact on the major depressive disorder therapeutics market. With ongoing research and clinical trials, these therapies hold the promise of reshaping major depressive disorder treatment, offering new hope to patients worldwide.
COMPASS Pathways’ COMP360
Phase – III
COMPASS Pathways is investigating its psilocybin-based therapy, COMP360, as a potential treatment for major depressive disorder and other serious mental health conditions. COMP360 is a synthetic formulation of psilocybin, administered with psychological support to enhance therapeutic outcomes. Psilocybin, the active compound found in certain “magic mushrooms,” has demonstrated promising antidepressant effects in clinical research. COMP360 has received Breakthrough Therapy Designation from the US FDA and Innovative Licensing and Access Pathway (ILAP) designation in the UK for treatment-resistant depression (TRD).
In November 2022, findings from a Phase IIb study on COMP360’s investigational psilocybin treatment were published in the New England Journal of Medicine. More recently, in April 2024, COMPASS Pathways and Journey Clinical announced a research collaboration aimed at developing a scalable delivery model and healthcare provider training program for COMP360. The company is also advancing a Phase II trial for MDD, while a Phase III trial is currently underway in partnership with Massachusetts General Hospital, further exploring COMP360’s potential in addressing unmet needs in depression treatment.
SAGE Therapeutics and Biogen’s Zuranolone
Phase – III
Zuranolone, developed by SAGE Therapeutics and Biogen, is a next-generation oral positive allosteric modulator designed for major depressive disorder treatment. It is a highly potent and selective compound that targets synaptic and extrasynaptic GABAA receptors, with binding sites distinct from those of GABA, benzodiazepines, and barbiturates. As the GABA system plays a crucial role in regulating CNS function, zuranolone’s mechanism offers a novel approach to treating MDD.
Currently in Phase III clinical development, it is being evaluated through the LANDSCAPE and NEST clinical trial programs, which include multiple studies assessing various dosing regimens, clinical endpoints, and treatment strategies in thousands of patients. In February 2023, the US FDA accepted the New Drug Application (NDA) for zuranolone as an MDD treatment. Under a collaboration agreement, Shionogi holds the rights to development and commercialization in Japan and selects other countries. However, in August 2023, the FDA issued a Complete Response Letter (CRL) for the NDA, prompting Sage and Biogen to review the feedback and evaluate their next steps in advancing the drug within the major depressive disorder therapeutics market.
Luye Pharma’s LY03005
Phase – III
LY03005, developed by Luye Pharma, is a key product in the company’s CNS therapeutic pipeline. It is an extended-release formulation of ansofaxine hydrochloride, a serotonin-norepinephrine-dopamine triple reuptake inhibitor (SNDRI) designed for major depressive disorder treatment. By targeting the reuptake of dopamine, serotonin, and norepinephrine, LY03005 aims to provide enhanced efficacy and fewer side effects compared to traditional SSRIs and SNRIs. As a prodrug of desvenlafaxine, it has demonstrated superior efficacy in clinical trials. In March 2020, Luye Pharma announced that the US FDA had reviewed and accepted the NDA filing for LY03005, marking a significant step in its development within the major depressive disorder therapeutics market.
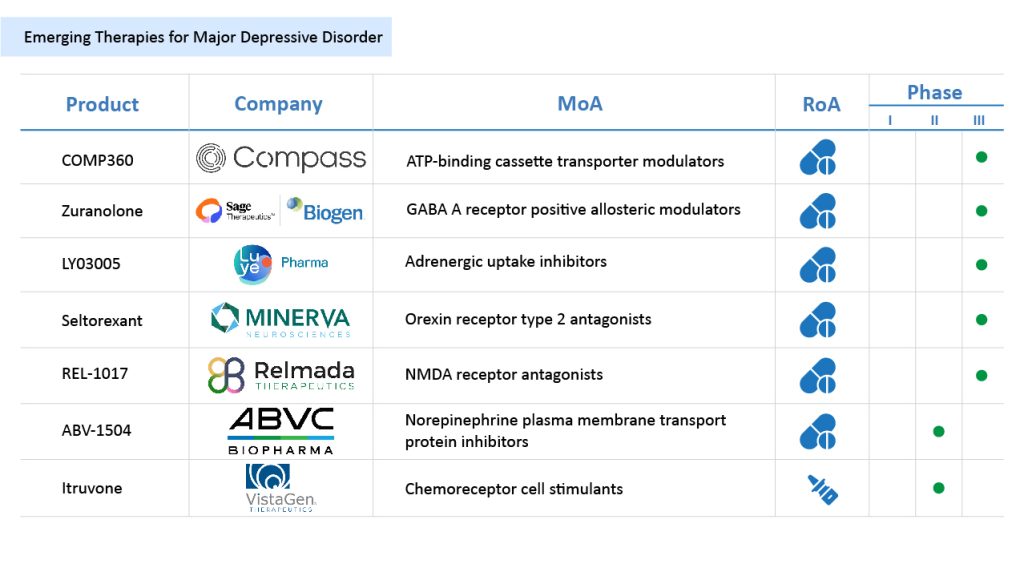
Minerva Neurosciences and Janssen Pharmaceutical’s Seltorexant
Phase – III
Seltorexant, developed by Minerva Neurosciences and Janssen Pharmaceutical, is a selective orexin-2 receptor (ORX2) antagonist designed as an adjunctive therapy for MDD. It is the most advanced ORX2-targeting molecule in clinical development, working by blocking orexin-2 receptors to enhance its antidepressant effects. In January 2021, Royalty Pharma acquired Minerva’s royalty interest in seltorexant for an upfront payment of USD 60 million, with the potential for up to USD 95 million in milestone payments. In certain indications, Minerva Neurosciences opted out of its joint development agreement with Janssen in 2020, retaining rights to mid-single-digit royalties on future global sales. In May 2024, Johnson & Johnson announced positive topline results from the pivotal Phase III MDD3001 trial, evaluating seltorexant as an adjunctive major depressive disorder treatment alongside baseline antidepressants in adults and elderly patients. These findings were presented at the American Society of Clinical Psychopharmacology (ASCP) Annual Meeting, highlighting the drug’s potential in the major depressive disorder therapeutics market.
Relmada Therapeutics’ REL-1017
Phase – III
REL-1017, developed by Relmada Therapeutics, is a new chemical entity (NCE) and a novel NMDA receptor (NMDAR) channel blocker designed to target hyperactive channels while preserving normal glutamatergic neurotransmission selectively. Recognized for its potential in major depressive disorder treatment, REL-1017 has received Fast Track Designation from the US FDA. In Phase II trials, the drug demonstrated rapid, robust, and sustained antidepressant effects, showing statistically significant improvements over placebo while maintaining a favorable safety, tolerability, and pharmacokinetics profile.
Currently, in late-stage development, REL-1017 is being evaluated in Phase III clinical trials as both an adjunctive and monotherapy for MDD treatment. However, in December 2024, Relmada Therapeutics announced the discontinuation of the Reliance II and Relight Phase III studies following a full dataset evaluation by the Data Monitoring Committee (DMC), despite previously published clinical data from the Reliance I Study in June 2024.
ABVC BioPharma’s ABV-1504
Phase – II
ABV-1504, developed by ABVC BioPharma, is a botanical investigational new drug containing PDC-1421 as its active ingredient. Through its subsidiary BioLite, ABVC has completed a Phase II clinical trial evaluating ABV-1504 as a Norepinephrine Transporter (NET) inhibitor for the treatment of major depressive disorder. The study at Stanford University confirmed that the PDC-1421 capsule was safe and well-tolerated, with low and high doses achieving the required 40% improvement in ADHD-RS-IV test scores.
In April 2023, PDC-1421 received a US Patent (US 11,554,154 B2) for its use in MDD treatment. Having successfully completed Phase II trials in major depressive disorder patients, ABVC BioPharma now plans to initiate an End of Phase II (EOP II) meeting with the FDA to advance the drug’s clinical development.
VistaGen Therapeutics’ Itruvone
Phase – II
Itruvone (PH10), developed by VistaGen Therapeutics, is an investigational first-in-class, fast-acting synthetic neurosteroid designed to address neuropsychiatric conditions associated with depression and suicidal ideation. VistaGen is initially advancing PH10 as a next-generation major depressive disorder treatment, offering a non-sedating, non-addictive, and self-administered alternative for patients. Administered as a nasal spray, PH10 delivers a microgram-level, non-systemic dose that activates nasal chemosensory receptors, triggering neural circuits in the brain to produce rapid antidepressant effects.
Unlike traditional major depressive disorder drugs, PH10 avoids systemic exposure and potential side effects linked to oral antidepressants and esketamine-based therapies. Having successfully completed Phase IIa trials, VistaGen is preparing for Phase IIb clinical development of PH10 for MDD treatment. Notably, in December 2022, the US FDA granted PH10 Fast Track designation, further accelerating its clinical advancement.
A New Era in Major Depressive Disorder Treatment
With the number of Major Depressive Disorder cases rising—the demand for innovative treatments has never been greater. The landscape of MDD treatment is evolving rapidly, with groundbreaking innovations in drug development offering new hope to patients worldwide. While traditional antidepressants like SSRIs and SNRIs remain widely used, the growing need for faster-acting, more effective, and well-tolerated therapies has driven pharmaceutical companies to explore novel mechanisms of action.
The emergence of next-generation treatments highlights the shift towards precision medicine and targeted therapies, addressing key challenges such as treatment-resistant depression, delayed onset of action, and adverse side effects. These advancements signal a transformative period in MDD therapeutics, where innovative approaches are reshaping the standard of care.
Beyond the drugs highlighted, numerous pharmaceutical companies—including Takeda Pharmaceuticals, Forest Laboratories, Otsuka Pharmaceuticals, Janssen Research & Development, Axsome Therapeutics, AbbVie, Praxis Precision Medicines, Chase Therapeutics, Neumora Therapeutics, BlackThorn Therapeutics, Fabre-Kramer Pharmaceuticals, Novartis, and many others—are actively driving innovation in the field.
With ongoing clinical trials, regulatory advancements, and increasing investments in mental health drug development, the future of MDD treatment looks promising. As these therapies progress through clinical and regulatory milestones, they have the potential to provide millions of patients worldwide with more effective, personalized, and accessible treatment options, offering a brighter path out of the fog of depression.

Downloads
Article in PDF
Recent Articles
- Samsung Partnered with Lunit; Boston Scientific Launched VersaVue single-use Flexible Cystoscope;...
- First oral GLP-1 treatment approved for type2 Diabetes
- FDA approves Ofev for interstitial lung disease
- FDA Approves Ascendis Pharma’s YORVIPATH; ARS Pharma Gets FDA Green Light for First Nasal Spray; ...
- FDA Approves Xolair for Food Allergies; FDA Accelerated Approval for Iovance’s AMTAGVI; Astellas ...



