APEX-AV is a single-arm Investigational Device Exemption (IDE) study that is accepting participants at up to 20 hospital-based sites across the United States who have verified acute, intermediate-risk PE. The APEX-AV Study’s main effectiveness outcome is the decrease in RV/LV ratio from baseline to 48 hours after the surgery. The rate of significant adverse events (MAEs), such as device-related death and major bleeding during the first 48 hours, is the main safety objective. In the 30 days following the index procedure, patients will be monitored.
“We are excited to have the first patient enrolled in this important trial as we assess the performance of the AlphaVac F1885 System in patients with intermediate-risk pulmonary embolisms,” states Juan Carlos Serna, AngioDynamics Senior Vice President of Clinical and Scientific Affairs. He added, “With our partners, we are demonstrating our continued commitment to generating robust clinical evidence with the potential to support our clinical and regulatory strategy to pursue additional indications to treat more patients and advance care.”
“We are privileged to have the first patient enrolled in this important PE trial using the AlphaVac F1885 System at Jacobi Medical Center. The procedure time was less than 60 minutes, and we were particularly impressed with the design, control, and navigability that the 18Fr cannula provides,” said Seth Sokol, MD, Cardiology Specialist at Jacobi Medical Center, and the site Principal Investigator.
As per DelveInsight’s “Pulmonary Embolism Market” report, the global pulmonary embolism market is expected to grow in the coming years owing to the increasing prevalence of pulmonary embolism, previous disease conditions, rising exposure to air pollution, chemicals, dust, and other, the increasing geriatric population across the globe, and the rise in smoking and tobacco use among youth among others.
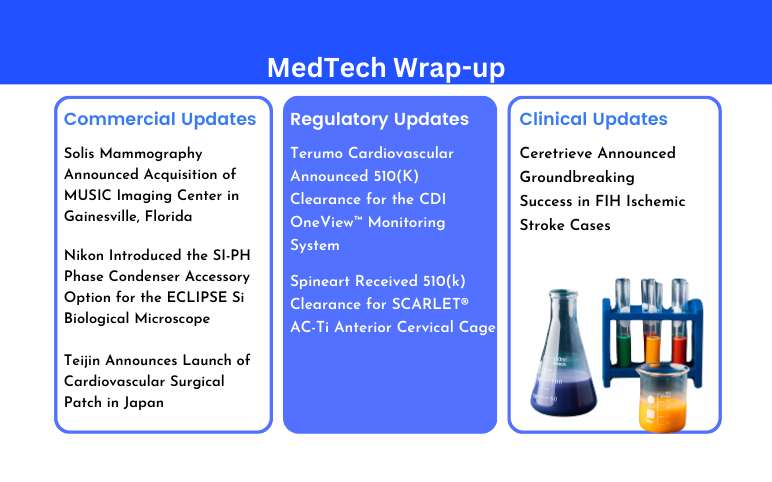
Abiomed to Get Acquired by Johnson & Johnson
On November 01, 2022, Johnson & Johnson, one of the world’s largest and diversified healthcare products company, will acquire through a tender offer all outstanding shares of Abiomed, who are pioneers in breakthrough heart, lung, and kidney support technologies broadening Johnson & Johnson MedTech’s (JJMT) position as a growing cardiovascular innovator.
As per the agreement between both companies, the acquisition will happen for an upfront payment of $380.00 per share in cash, corresponding to an enterprise value of approximately $16.6 billion, which includes cash acquired.
The shareholders of Abiomed will also receive a non-tradable contingent value right (CVR) which entitles the holder to receive up to $35.00 per share in cash if certain commercial and clinical milestones are achieved. The boards of directors from both companies unanimously approved the transaction.
Joaquin Duato, Chief Executive Officer of Johnson & Johnson, said, “The addition of Abiomed is an important step in the execution of our strategic priorities and our vision for the new Johnson & Johnson focused on Pharmaceutical and MedTech.” He further added, “We have committed to enhancing our position in MedTech by entering high-growth segments. The addition of Abiomed provides a strategic platform to advance breakthrough treatments in cardiovascular disease and helps more patients around the world while driving value for our shareholders.”
According to DelveInsight’s “Cardiovascular Ultrasound System Market” report, the global cardiovascular ultrasound system market was valued at USD 1.61 billion in 2021, growing at a CAGR of 4.79% during the forecast period from 2022 to 2027, to reach USD 2.11 billion by 2027. The cardiovascular ultrasound system market is estimated to register positive revenue growth owing to the factors such as the rising prevalence of cardiovascular diseases globally coupled with the rising geriatric population. Moreover, the increase in sedentary lifestyles and the increasing focus of manufacturers to develop technologically advanced cardiovascular ultrasound systems are likely to propel the market of cardiovascular ultrasound systems in the upcoming years.
FDA Approves the Cochlear™ Nucleus® 8 Sound Processor
On November 1, 2022, Cochlear Limited, pioneers in implantable hearing solutions, received the U.S. Food and Drug Administration (FDA) approval for the Cochlear™ Nucleus® 8 Sound Processor.
The behind-the-ear cochlear implant sound processor Nucleus 8 Sound Processor is currently the smallest and lightest model on the market. Additionally, it is the first cochlear implant sound processor in the world equipped with the most recent Bluetooth® LE Audio technology, enabling direct audio connectivity to common consumer gadgets.
Cochlear’s newest and most inventive hearing technology, which can detect changes in a person’s environment and automatically adapt their listening settings, is featured in the Nucleus 8 Sound Processor. Additionally, it has an improved ForwardFocus feature that, especially in noisy surroundings, efficiently eliminates background noise to make face-to-face talks more understandable.
“Cochlear’s goal is to help people feel empowered to connect with their world and make it easier to hear and be heard,” commented Lisa Aubert, President of Cochlear Americas. She further added, “In a restaurant, at work, at school, or spending time with family and friends – life’s full of opportunities to engage with others. The Nucleus 8 Sound Processor is our smallest, lightest and smartest behind-the-ear sound processor yet and is designed to help people hear conversations more clearly and comfortably, wherever life may take them.”
According to DelveInsight’s “Cochlear Implants Market” report, the global cochlear implants market was valued at USD 1.52 billion in 2021, growing at a CAGR of 9.92% during the forecast period from 2022 to 2027, to reach USD 2.68 billion by 2027. The increase in demand for cochlear implants is predominantly attributed to the rising prevalence of hearing loss of different types, the increasing geriatric population, and the rising number of technological advancements in cochlear implants, among others.
Pfizer Announces Positive Top-Line Data of Phase III Global Maternal Immunization Trial for its Bivalent Respiratory Syncytial Virus (RSV) Vaccine Candidate
On November 01, 2022, Pfizer Inc. announced that it received positive top-line data from the Phase III clinical trial MATISSE (Maternal Immunization Study for Safety and Efficacy) investigating its bivalent RSV prefusion vaccine candidate, RSVpreF or PF-06928316 when administered to pregnant participants in order to protect their infants from RSV disease after birth.
In newborns from birth through the first 90 days of life, a vaccine efficacy of 81.8% against severe medically attended lower respiratory tract illnesses caused by RSV was seen. A high efficacy of 69.4% was also seen through the first six months of life.
Both those who received the RSVpreF experimental vaccine and their newborns tolerated it well with no safety issues.
Also, the results satisfied one of the predetermined regulatory success criteria in the trial protocol, and Pfizer intends to submit its first regulatory application by the end of 2022.
The RSV vaccine candidate from Pfizer, if approved, might be the first maternal vaccine available to help prevent this frequent and possibly fatal respiratory infection in early infants.
Pfizer is presently the sole business working on regulatory applications for an experimental vaccine to help protect against RSV in both older adults and infants through maternal immunization.
“We are thrilled by these data as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” said Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research & Development, Pfizer. She added, “These data reinforce Pfizer’s resolve to bring our expertise in the research and development of innovative vaccines to address critical public health needs using new approaches and technologies. We look forward to working with the FDA and other regulatory agencies to bring this vaccine candidate to expectant mothers to help protect their infants against severe RSV during their most vulnerable first six months of life, which has the highest burden of RSV illness in infants. We would like to thank the pregnant women who volunteered for this trial, along with their infants, and all the investigators around the world who participated in the study for their contribution to this landmark research.”
According to DelveInsight’s “Vaccines Market” report, the global vaccines market will grow at a CAGR of –11.47% during the forecast period from 2022 to 2027 to reach USD 65,074.86 million by 2027. The vaccines market is witnessing positive market growth owing to the factors such as the rising prevalence of cancers such as liver, cervical, and others that are contributing to the growing demand for therapeutics and prophylactic cancer vaccines. Moreover, the growing focus on reducing childhood morbidity and mortality rates due to preventable diseases in children is another prominent factor responsible for the growth of the vaccine market. Additionally, the COVID-19 pandemic also presented opportunities for the growth of the vaccine market.
Intelligent Ultrasound Received FDA Clearance for ScanNav Anatomy Peripheral Nerve Block (PNB)
On October 20, 2022, Intelligent Ultrasound, a ‘classroom to clinic’ ultrasound company specializing in artificial intelligence (AI) software and simulation, received De Novo clearance from the U.S. Food & Drug Administration (FDA) for clinical use of its AI medical device, ScanNav Anatomy Peripheral Nerve Block (PNB).
With the help of AI technology, ScanNav Anatomy PNB is designed to assist healthcare professionals in identifying and labeling anatomy in live ultrasound images in preparation for ultrasound-guided regional anesthesia. It is capable of highlighting the anatomy associated with nine common peripheral nerve blocks.
Ultrasound-guided peripheral nerve block injections used for pain relief after surgery and trauma or as a prudent alternative to general anesthesia are difficult to deliver. ScanNav Anatomy can help in the easy delivery of ultrasound-guided nerve blocks.
The company is willing to launch ScanNav Anatomy very soon in the US market, and it is going to be sold as a stand-alone device that can be plugged into compatible general-purpose diagnostic ultrasound systems.
The device has been classified by FDA under the de novo program indicating that there are no devices already on the market which meet the same clinical need.
“We’re delighted to have received the FDA De Novo grant for our second AI product, and to build on the success of ScanNav Assist, our obstetric AI software. ScanNav Anatomy PNB will launch into the US anaesthesiology ultrasound market, continuing the expansion of our AI-based real-time clinical ultrasound image analysis software. Although all new medical markets take time to develop, we are excited about the long-term potential of our increasing range of AI image analysis products,” commented Stuart Gall, CEO of Intelligent Ultrasound Group plc.
According to DelveInsight’s “Anaesthesia Workstations/Machines Market” report, the global anesthesia workstations/machines market is estimated to advance at a CAGR of 8.2% during the forecast period from 2022 to 2027. The demand for anesthesia workstations/machines is primarily attributed to the growing burden of various surgeries that require anesthesia for the patients during the operation. In addition, the increasing prevalence of chronic diseases, growing old age population, and increase in investments and funds for the development of anesthesia workstations/machines by the key players are also likely to accelerate the market demand for anesthesia workstations/machines during the forecast period.
Ameda Launches the Ameda Pearl™ Breast Pump
On October 26, 2022, Ameda, one of the manufacturers of breast pumps, announced the launch of their newest hospital-grade breast pump, named Ameda Pearl™, designed to support moms in the hospital and at home.
Pearl is a portable, durable, and multi-user pump that comes with integrated bottle holders and also has easy-to-clean housing. It offers hospital-grade pumping through two modes of pumping and a wide range of adjustable settings.
Pearl is compatible with the Ameda Universal HygieniKit™, which is the world’s only FDA-cleared pump kit that helps protect breastmilk from harmful contaminants.
“The Ameda Pearl was designed with the pump dependent mom in mind,” Susan Rappin, Vice President of Marketing, commented. She added, “Moms appreciate the thoughtful details that make frequent pumping easier – a long-lasting rechargeable battery, an LCD screen with timer, whisper quiet operation and even an adjustable night-light for late night sessions.”
According to DelveInsight’s “Breast Pumps Market” report, the global breast pump market was valued at USD 1,081.96 million in 2021, growing at a CAGR of 8.67% during the forecast period from 2022 to 2027 to reach USD 1,776.50 million by 2027. Factors such as the surge in the number of employed mothers globally, technological advancements in the product offering, changes in consumer lifestyle, and increasing awareness about breast pumps, among others, are going to drive the market for breast pumps. As per DelveInsight’s study, among all the regions, North America is expected to hold the highest market share. This is primarily attributed to a large number of employed mothers in the region and the increasing product launches for the breast pump market.
Thermo Fisher Scientific to Acquire the Binding Site Group for USD 2.6 billion
On October 31, 2022, Thermo Fisher Scientific, the world leader in serving science, is planning to acquire The Binding Site Group, which is a specialty diagnostic assays and instruments company.
The Binding Site provides specialty diagnostic assays and instruments to improve the diagnosis and management of blood cancers and immune system disorders. The Binding Site’s Freelite® offering is widely recommended for multiple myeloma diagnosis and monitoring across all stages of the disease by major clinical guideline publications.
The Binding Site, headquartered in Birmingham, United Kingdom, employs over 1,100 people worldwide and is an active and influential member of the scientific community. The Binding Site has an appealing financial profile as an established leader in a fast-growing segment where patient care has shifted toward early diagnosis and monitoring via regular testing. Its business has been growing at a rate of about 10% per year and is on track to generate more than $220 million in revenue by 2022. The Binding Site’s strong clinical value enables doctors worldwide to support millions of patients each year.
A shareholder group formed by the European private equity firm Nordic Capital has agreed to sell The Binding Site to Thermo Fisher. It places a $2.6 billion value on the cash-only transaction.
“This transaction perfectly aligns with our mission and is an exciting addition to our existing specialty diagnostic offerings. With extensive expertise and a large and dedicated installed base in cancer diagnostics, The Binding Site will further enhance our specialty diagnostics portfolio,” said Marc N. Casper, chair, president, and CEO of Thermo Fisher. He added, “The Binding Site is extremely well-respected by researchers and clinicians alike for its pioneering diagnosis and monitoring solutions for multiple myeloma.”
According to DelveInsight’s “Point of Care Diagnostics Market” report, the point of care diagnostics market was valued at USD 1.85 billion in 2021, growing at a CAGR of 7.99% during the forecast period from 2022 to 2027 to reach USD 2.93 billion by 2027. Factors such as the increasing prevalence of diabetes, rising number of patients suffering from infectious diseases, increasing frequency of both acute and chronic diseases, and technological advancements in the product offering, among others, are going to drive the market for point-of-care diagnostics during the forecast period from 2022 to 2027.