Mucopolysaccharidosis Type I Treatment Pipeline — 4 Promising Therapies on the Way
Mar 31, 2025
Table of Contents
MPS I treatment typically involves Hematopoietic Stem Cell Transplantation (HSCT), Enzyme Replacement Therapy (ERT), or a combination of both, along with supportive care such as pain management, anti-inflammatory medications, oxygen therapy, and surgery. While these MPS I treatments help manage symptoms and improve survival, they cannot reverse existing damage. Early intervention is essential to achieve the best possible outcomes.
ALDURAZYME (developed by BioMarin and Sanofi) is the only therapy approved by the US FDA for treating MPS I. ALDURAZYME was approved in the US and EU4 (France, Germany, Italy, and Spain) and the UK in 2003 and in Japan in 2006. It is indicated for patients with the Hurler and Hurler-Scheie forms of MPS I, as well as those with the Scheie form who have moderate to severe symptoms. The treatment includes a boxed warning about potential risks.
Downloads
Article in PDF
Although its patent has expired, no biosimilars are currently available, making ALDURAZYME the only enzyme replacement therapy for MPS I on the market. However, several companies are working with their lead assets that will give tough competition to BioMarin/Sanofi’s ALDURAZYME.
4 Promising MPS I Therapies in Development
The MPS I treatment pipeline remains limited, with only a few new therapies currently in development. Leading companies driving progress include Kyowa Kirin and Orchard Therapeutics (developing OTL-203, a gene therapy targeting the underlying enzyme deficiency), JCR Pharmaceuticals (advancing JR-171, an enzyme replacement therapy that can cross the blood-brain barrier), Immusoft (developing ISP-001, a cell-based platform for continuous enzyme production), and REGENXBIO (working on RGX-111, a gene therapy designed to deliver a functional copy of the IDUA gene).
These innovative treatments aim to improve neurological function and overall patient outcomes, addressing critical unmet needs in the MPS I community. Let’s get into detail about these 4 MPS I therapies in development.
Orchard Therapeutics/Kyowa Kirin’s OTL-203
OTL-203 is a one-time gene therapy for MPS IH that uses a patient’s own hematopoietic stem and progenitor cells (HSPCs). These cells are collected from mobilized peripheral blood and genetically modified ex vivo with a lentiviral vector carrying functional IDUA complementary DNA to restore enzyme production and reduce GAG accumulation. Developed in collaboration with the San Raffaele-Telethon Institute for Gene Therapy (SR-Tiget), OTL-203 is currently in Phase III MPS I clinical trials in North America and Europe.
Orchard Therapeutics plans to submit a US application in 2028, with potential approval expected in 2029 under priority review. OTL-203 has received Fast Track Designation (FTD), Orphan Drug Designation (ODD), and Rare Pediatric Disease Designation (RPDD) from the FDA, as well as PRIME status from the EMA.
At the 21st Annual WORLDSymposium in February 2025, Orchard Therapeutics presented updated findings from a proof-of-concept study on OTL-203, highlighting improvements in neurological, skeletal, and other clinical outcomes. Additionally, in February 2024, the company announced the first patient’s randomization in the registrational trial of OTL-203 for MPS IH, marking a key milestone in its clinical development.
JCR Pharmaceuticals’ JR-171
JR-171 (lepunafusp alfa) is an advanced enzyme replacement therapy (ERT) designed to address central nervous system (CNS) complications in MPS I. It is a recombinant fusion protein that combines an antibody targeting the human transferrin receptor with IDUA, the deficient enzyme in MPS I patients. By leveraging transferrin receptor-mediated transcytosis, JR-171 effectively crosses the blood-brain barrier (BBB), addressing a critical gap in CNS treatment.
Developed using JCR Pharmaceuticals’ proprietary J-Brain Cargo and J-MIG System platforms, JR-171 has completed a 13-week Phase I/II clinical trial in Japan and the US, with an extension study currently underway. JR-171 is being developed through licensing partnerships, with ongoing collaboration discussions. It has received Fast Track Designation (FTD) and Orphan Drug Designation (ODD) from both the FDA and EMA.
In September 2024, JCR Pharmaceuticals presented data at the Society for the Study of Inborn Errors of Metabolism (SSIEM) Annual Symposium, highlighting improvements in both neurobehavioral and somatic symptoms in MPS I patients treated with JR-171.
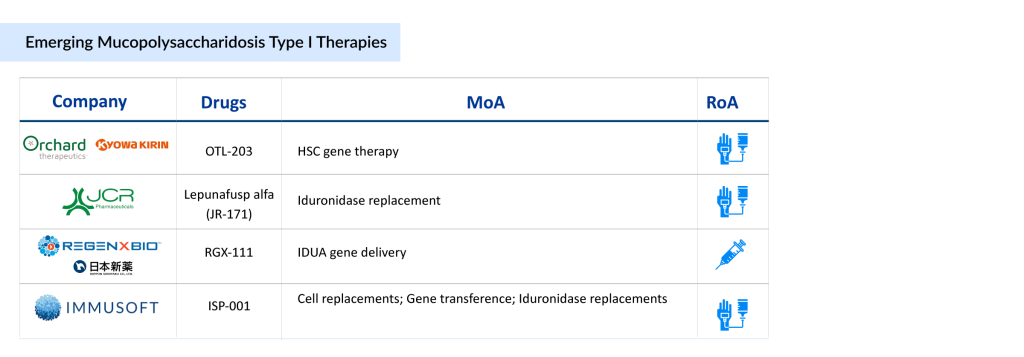
REGENXBIO/Nippon Shinyaku’s RGX-111
RGX-111 is an investigational gene therapy designed to treat MPS I, a rare genetic disorder caused by a deficiency of the IDUA enzyme. It works by delivering a functional copy of the IDUA gene directly to the central nervous system (CNS), enabling brain cells to produce the enzyme. This approach aims to slow or prevent cognitive decline and neurological damage associated with MPS I.
Interim results from an ongoing Phase I/II trial have shown encouraging biological activity and a favorable safety profile. Recognized for its potential, RGX-111 has been granted Orphan Drug Designation (ODD), Rare Pediatric Disease Designation (RPDD), and Fast Track Designation (FTD) by the US FDA for mucopolysaccharidosis type I treatment.
Recently, in March 2025, REGENXBIO Inc. finalized its previously announced strategic partnership with Nippon Shinyaku. Under this agreement, the two companies will collaborate on the development and commercialization of RGX-111 for mucopolysaccharidosis type I, also known as Hurler syndrome, in both the United States and Asia.
Immusoft’s ISP-001
ISP-001 is an autologous B cell therapy engineered to produce human alpha-L-iduronidase for treating MPS I (Hurler syndrome). The drug is currently being tested in a Phase I MPS I clinical trial. It received Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPDD) from the US FDA in 2018 for MPS I. In January 2025, Immusoft announced encouraging results from the first human trial of ISP-001, which will be presented at the WORLDSymposium 2025 in San Diego.
What Lies Ahead in MPS I Treatment?
The future of MPS I treatment beyond ERT is promising, with advancements in gene therapy, substrate reduction therapy, and small molecule treatments offering new hope for better outcomes. While ERT, such as laronidase, has been effective in addressing some systemic symptoms, it is limited by its inability to cross the blood-brain barrier and fully address neurological complications. Future therapies aim to overcome these limitations by delivering more targeted and long-lasting effects.
Gene therapy for MPS I treatment is emerging as a transformative approach, with the potential to provide a one-time treatment that addresses the underlying genetic defect. AAV and lentiviral vectors are being explored to deliver functional copies of the defective gene, potentially enabling sustained production of the missing enzyme. Early-stage MPS I clinical trials have shown encouraging results, with reduced GAG accumulation and improved cognitive function. Additionally, gene editing techniques such as CRISPR-Cas9 hold promise for precise correction of the genetic mutation, potentially offering a permanent cure.
Beyond gene therapy, novel approaches such as substrate reduction therapy (SRT) and chaperone therapy are being developed to improve cellular metabolism and enhance the stability and activity of endogenous enzymes. Intrathecal and intracerebroventricular delivery methods are also being tested to enhance CNS penetration and address neurological symptoms more effectively. Combined therapies, including ERT alongside gene therapy or SRT, could provide a more comprehensive treatment strategy. These advances have the potential to significantly improve both the quality of life and life expectancy for MPS I patients.

Downloads
Article in PDF



