Revolutionizing Multiple Sclerosis Treatment: A New Era of Therapies
Jul 24, 2023
Table of Contents
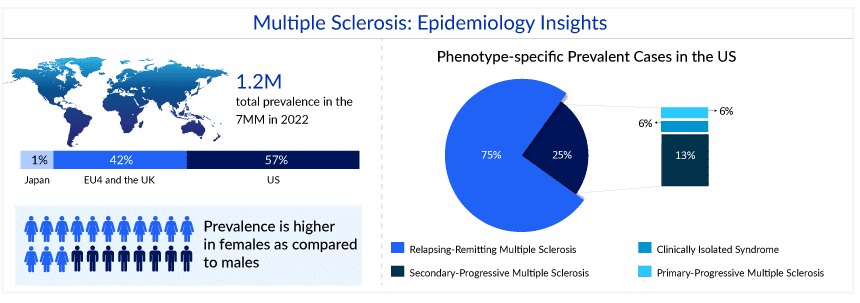
Multiple sclerosis is an unpredictable, autoimmune disease that affects the central nervous system. Multiple sclerosis is believed to impact almost 1 million Americans, and the disease manifests itself in a variety of ways. Because nerves are affected in different ways and exhibit a variety of symptoms, no two cases of multiple sclerosis, a disease that affects the central nervous system, are the same. Women are more likely to be diagnosed with multiple sclerosis than men, with a ratio of about 3:1.
As per DelveInsight’s estimates, the total 7MM prevalent cases of multiple sclerosis in 2022 were approximately 1.2 million cases. The cases are expected to increase by 2032. Assessments as per DelveInsight’s analysts showed that in 2022, in the United States, out of the total cases, there were approximately 41K cases with Clinically Isolated Syndrome, 545K cases with Relapsing-Remitting MS (RRMS), 92K cases with Secondary-Progressive MS (SPMS), and 44K cases with Primary-Progressive MS (PPMS).
Downloads
Article in PDF
Recent Articles
- The Growing Burden Of Neurodegenerative Disorders
- Abbott’s Alinity m STI Assay; Phillips’ MR 7700 System; HOYA’s MiYOSMART Spectacle Lens; Magneto’...
- CanSino deals with Precision; Atea raises USD 215 M; Applied Molecular Transport targets for USD ...
- BMS, and Exelixis’s Opdivo + CABOMETYX in First-Line Advanced Renal Cell Carcinoma; AIRSUPRA Now ...
- Allumiqs and Prolytix’s Partnership; Setpoint Medical’s Neuroimmune Modulation Platform get...
Navigating the Current Multiple Sclerosis Treatment Landscape: What Patients Need to Know
There is no cure for multiple sclerosis, although there are numerous multiple sclerosis treatment options that enhance patients’ long-term outcomes. Most people will deteriorate if they do not receive multiple sclerosis treatment. The goal of multiple sclerosis treatment is to decrease relapses and enhance and maintain functionality throughout the patient’s life. Multiple sclerosis treatment consists of two components: DMT for the underlying immunological condition and therapies to relieve or change symptoms. Disease-modifying agents aim to reduce relapses and decrease progression. DMT is only licensed for treatment in relapsing forms of multiple sclerosis. Persistence was similarly poor among multiple sclerosis patients who switched DMTs, independent of therapy. Although oral DMTs were marginally more persistent than injectable DMTs, overall results show poor persistence to second-line therapy and underscore the need to improve long-term DMT persistence.
Last year in December, TG Therapeutics’ BRIUMVI has been approved by the FDA for the treatment of relapsing forms of multiple sclerosis (RMS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. Approval for this indication was granted based on data from the ULTIMATE I & II Phase III trials, which demonstrated that it was superior to teriflunomide in significantly lowering the annualized relapse rate (ARR, the primary endpoint), the number of T1 Gd-enhancing lesions, and the number of new or enlarging T2 lesions. The results of the ULTIMATE I and II trials were published in The New England Journal of Medicine in August 2022.
Cycle Pharmaceuticals recently announced the launch of TASCENSO ODT, its first medicine for treating multiple sclerosis patients in the United States. The launch assures that multiple sclerosis patients in the United States who are currently receiving GILENYA, or generic fingolimod, have access to appropriate patient support services in addition to the bioequivalent, non-generic, TASCENSO ODT. GILENYA patient support services are slated to be phased out on March 31, 2023. The US FDA approves ASCENSO ODT for the treatment of relapsing forms of multiple sclerosis, which include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 years of age and older, and is available in 0.25 mg and 0.5 mg doses.
Additionally, with continuous efforts in research and development, various companies are developing therapies that will change the multiple sclerosis treatment dynamics in the coming years.

Emerging Therapies for Multiple Sclerosis Treatment: A Promising Outlook
Several pharmaceutical and biotechnology companies are actively working on developing therapies for multiple sclerosis treatment. NeuroVax (Immune Response BioPharma), IMS001 (ImStem Biotechnology/Rho), CNM-Au8 (Clene Nanomedicine), Tolebrutinib (SAR442168) (Sanofi), Evobrutinib/M2951 (Merck Healthcare KGaA), ATA188 (Atara Biotherapeutics), ANK-700 (Anokion SA), and others, are the promising emerging therapies that are in various stages of clinical development in multiple sclerosis pipeline.
NeuroVax (Immune Response BioPharma) is a therapeutic TCR (T-cell receptor) peptide vaccine for secondary progressive multiple sclerosis (SPMS). In March 2020, the company initiated a Phase II/III, multi-center, randomized, double-blind, placebo-controlled, two-arm parallel design study of NeuroVax vs. Incomplete Freund’s Adjuvant (IFA) placebo with Secondary Progressive SPMS. The study is estimated to complete on March 2023, with an estimated enrollment of 200 participants. However, the company is providing no updates on the drug. The vaccine is also being studied in Phase I for pediatric multiple sclerosis. In March 2014, NeuroVax Granted Fast Track FDA Designation for SPMS.
Tolebrutinib, also known as SAR442168 is being developed by Sanofi. It is a Bruton tyrosine kinase (BTK) inhibitor, with IC50 values of 0.4 nM and 0.7 nM in Ramos B cells and HMC microglia cells, respectively. Tolebrutinib blocks the BCR-mediated activation (IC50 = 10 nM) and Fc receptor activation (IC50 = 166 and 9.6 nM for FcεR and FcγR, respectively) of immune cells. Tolebrutinib inhibits microglial FcγR activation through durable occupancy of BTK, with an IC50 of 157 nM. The drug is being studied in three Phase III trials for the treatment of relapsing multiple sclerosis, PPMS, and SPMS.
Evobrutinib/M2951 (Merck Healthcare KGaA) is an oral, highly selective inhibitor of Bruton’s tyrosine kinase (BTK) in clinical development as a potential multiple sclerosis treatment. It is the first BTK inhibitor to demonstrate clinical efficacy in the largest Phase II study with follow-up beyond two years as well as demonstrate an impact on early biomarkers of chronic inflammation that correlate with disease progression. Evobrutinib is designed to inhibit primary B cell responses such as proliferation and antibody and cytokine release, without directly affecting T cells. The drug is being studied in Phase III studies for the treatment of relapsing multiple sclerosis.
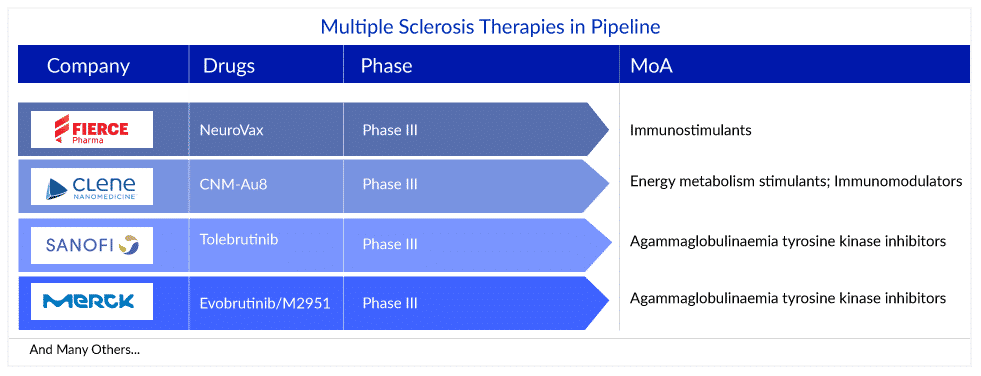
CNM-Au8 (Clene Nanomedicine) is a concentrated, aqueous suspension of clean surfaced faceted nanocrystalline gold (Au) that acts catalytically to support various intracellular biological reactions. CNM-Au8 consists solely of gold atoms organized into faceted, geometrical crystals held in suspension in sodium bicarbonate buffered, pharmaceutical-grade water. In October 2019, Clene Nanomedicine, Inc., announced that the National Multiple Sclerosis Society (NMSS) had awarded the company a research grant totaling more than 339,000 USD to support the clinical development of its nanocatalytic therapeutic, CNM-Au8, for the treatment of multiple sclerosis. The drug is currently being evaluated in Phase II studies for the treatment of RRMS.
Recent Developments in the Multiple Sclerosis Treatment Space
- In July 2023, Synaptogenix, Inc., an emerging biopharmaceutical company exploring regenerative therapies for neurodegenerative diseases, signed an agreement with Cleveland Clinic to perform a phase 1 trial of Bryostatin-1 in multiple sclerosis.
- In July 2023, Roche revealed positive findings from a late-stage study of Ocrevus (ocrelizumab) as a twice-yearly subcutaneous injection in patients with relapsing types of multiple sclerosis or primary progressive multiple sclerosis.
- In June 2023, the European Commission approved Briumvi (ublituximab) for the treatment of people with active relapse multiple sclerosis, as defined by clinical or imaging characteristics.
- In April 2023, the FDA temporarily halted clinical trials of evobrutinib, Merck KGaA’s investigational BTK inhibitor for relapsing forms of multiple sclerosis.
Shaping the Future of Multiple Sclerosis Treatment
The future outlook of multiple sclerosis treatment holds promise for patients worldwide as advancements in medical research and technology pave the way for more effective and personalized therapies. Over the past decades, significant progress has been made in understanding the complex nature of multiple sclerosis, enabling the development of novel multiple sclerosis treatments that target the immune system and repair mechanisms of the nervous system. As we venture into the future, several key trends are expected to define the landscape of multiple sclerosis treatment.
Firstly, the emergence of precision medicine is set to revolutionize multiple sclerosis treatment approaches. Genetic profiling and biomarker analysis will enable clinicians to tailor therapies to individual patients, ensuring better outcomes and minimizing side effects. Additionally, a deeper understanding of the disease’s underlying mechanisms may lead to the discovery of new targets for drugs, further enhancing multiple sclerosis treatment options.
Secondly, innovative therapies, including gene and cell-based therapies, hold great potential for halting or even reversing the progression of multiple sclerosis. Techniques like gene editing and stem cell transplantation have already shown promise in preclinical studies, and ongoing multiple sclerosis clinical trials are expected to shed light on their safety and efficacy in human patients.
Thirdly, the integration of digital health solutions will transform the way multiple sclerosis is managed. Wearable devices and mobile applications will enable real-time monitoring of symptoms, disease progression, and treatment responses, empowering both patients and healthcare providers to make informed decisions and adjust multiple sclerosis therapies as needed.
Moreover, collaborative research efforts across the globe will facilitate knowledge exchange and accelerate breakthroughs in multiple sclerosis treatment. International partnerships and data-sharing initiatives will enable researchers to pool resources and better understand the disease’s complexities, fostering a more comprehensive approach to managing multiple sclerosis.
FAQs
Multiple sclerosis is a chronic neurological disorder that affects the central nervous system. It is characterized by the immune system mistakenly attacking the protective covering of nerve fibers, known as myelin, leading to communication problems between the brain and the rest of the body.
The symptoms of multiple sclerosis can vary widely from person to person and depend on the location and extent of nerve damage. Some common multiple sclerosis symptoms include fatigue, difficulty walking, numbness or tingling in the limbs, muscle weakness, problems with coordination and balance, blurred or double vision, dizziness, and cognitive impairment.
Diagnosing multiple sclerosis can be challenging as there is no specific test that can confirm the presence of the disease definitively. Instead, doctors rely on a combination of medical history, neurological examinations, and diagnostic tests.
Although there is no known cure for multiple sclerosis, there are various treatment options available to manage the symptoms and slow down the progression of the disease. Multiple sclerosis treatment approaches typically focus on three main aspects: modifying the disease course, managing symptoms, and providing supportive care.

Downloads
Article in PDF
Recent Articles
- Virus, the Cancer Therapy of the Future
- Notizia
- Biogen buys Nightstar; Pacira acquires Myoscience; Horizon Pharma announces pricing; STAT with Sl...
- Mylan closing Illinois plant; Insys spends $24M; Roche’s Ocrevus; Greece’s corruption prose...
- 6 Emerging Treg Cell-based Therapies Shaping the Future of Immunotherapy



